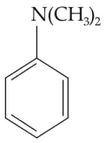
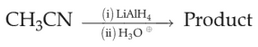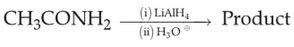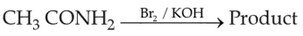Home >> Topics >> Amines
Unattempted Questions
Questions Available: 12
Solution
Year: 2025
Topic: Amines
2.
Given below are two statements :
Statement I : Benzenediazonium salt is prepared by the reaction of aniline with nitrous acid at 273 - 278 K. It decomposes easily in the dry state.
Statement Il : Insertion of iodine into the benzene ring is difficult and hence iodobenzene is prepared through the reaction of benzenediazonium salt with KI.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Benzenediazonium salt is prepared by the reaction of aniline with nitrous acid at 273 - 278 K. It decomposes easily in the dry state.
Statement Il : Insertion of iodine into the benzene ring is difficult and hence iodobenzene is prepared through the reaction of benzenediazonium salt with KI.
In the light of the above statements, choose the most appropriate answer from the options given below :
Solution
Year: 2024
Topic: Amines
3.
Given below are two statements:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
Solution
Solution
Solution
Year: 2022
Topic: Amines
6.
Given below are two statements :
Statement I : Primary aliphatic amines react with HNO2 to give unstable diazonium salts.
Statement Il : Primary aromatic amines reactwith HNO2 to form diazonium salts which are stable even above 300 K.
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement I : Primary aliphatic amines react with HNO2 to give unstable diazonium salts.
Statement Il : Primary aromatic amines reactwith HNO2 to form diazonium salts which are stable even above 300 K.
In the light of the above statements, choose the most appropriate answer from the options given below: