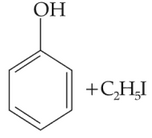
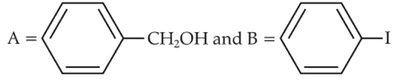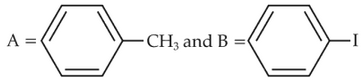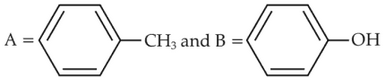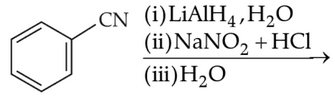Home >> Topics >> Alcohols, Phenols and Ethers
Unattempted Questions
Questions Available: 20
Solution
Solution
Solution
Solution
Solution
Solution
Solution
Solution
Year: 2022
Topic: Alcohols, Phenols and Ethers
9.
Given below are two statements:
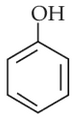
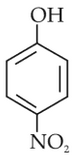
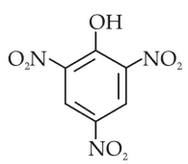
Statement I: The acidic strength of monosubstituted nitrophenol is higher than phenol because of electron withdrawing nitro group.
Statement II : O-nitrophenol, m-nitrophenol and P-nitrophenol will have same acidic strength as they have one nitro group attached to the phenolic ring.
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
Statement I: The acidic strength of monosubstituted nitrophenol is higher than phenol because of electron withdrawing nitro group.
Statement II : O-nitrophenol, m-nitrophenol and P-nitrophenol will have same acidic strength as they have one nitro group attached to the phenolic ring.
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
Solution
Year: 2022
Topic: Alcohols, Phenols and Ethers
10.
Given below are two statements:
Statement I : In Lucas test, primary, secondaryand tertiary alcohols are distinguished on the basisof their reactivity with conc. HCl + ZnCl2, known as Lucas Reagent.
Statement II : Primary alcohols are most reactive and immediately produce turbidity at roomtemperature on reaction with Lucas Reagent.
In the light of the above statements, choose the mostappropriate answer from the options given below:
Statement I : In Lucas test, primary, secondaryand tertiary alcohols are distinguished on the basisof their reactivity with conc. HCl + ZnCl2, known as Lucas Reagent.
Statement II : Primary alcohols are most reactive and immediately produce turbidity at roomtemperature on reaction with Lucas Reagent.
In the light of the above statements, choose the mostappropriate answer from the options given below:

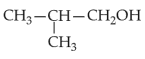
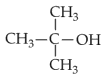
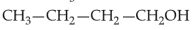
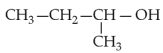
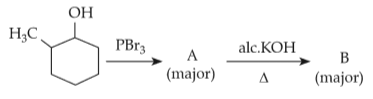
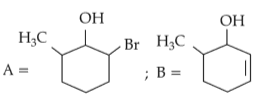
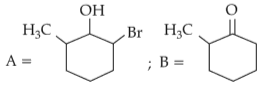
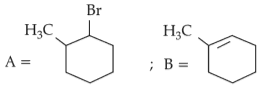
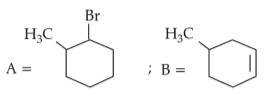
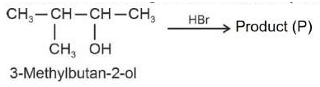
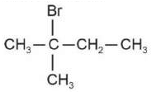

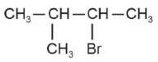
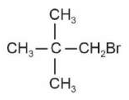
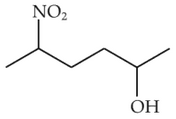
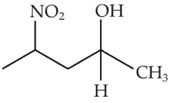
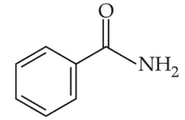
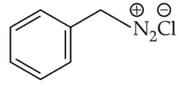
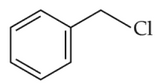
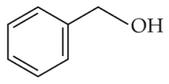
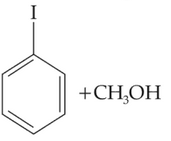
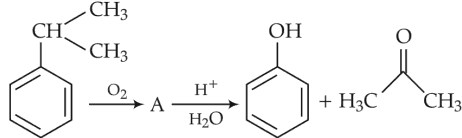
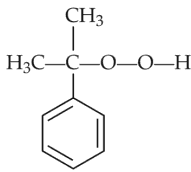
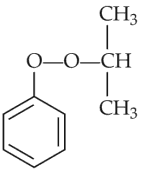
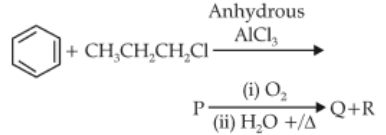
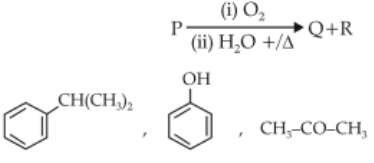
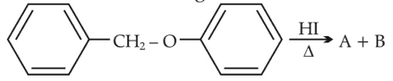 Identify products A and B:-
Identify products A and B:-