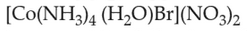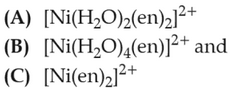Home >> Topics >> Coordination Compounds
Unattempted Questions
Questions Available: 26
Year: 2024
Topic: Coordination Compounds
1.
Given below are two statements:
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
Solution
Solution
Solution
Solution
Solution
Solution
Year: 2025
Topic: Coordination Compounds
7.
The correct order of the wavelength of light absorbed by the following complexes is,
A. \([Co\left(NH_3\right)_6]^{3+}\)
B. \([Co\left(CN\right)_6]^{3-}\)
C. \([Cu\left(H_2O\right)_4]^{2+}\)
D. \([Ti \left(H_2O \right)_6 ]^{3+}\)
Choose the correct answer from the options given below:
A. \([Co\left(NH_3\right)_6]^{3+}\)
B. \([Co\left(CN\right)_6]^{3-}\)
C. \([Cu\left(H_2O\right)_4]^{2+}\)
D. \([Ti \left(H_2O \right)_6 ]^{3+}\)
Choose the correct answer from the options given below:
Solution
Solution
Year: 2025
Topic: Coordination Compounds
9.
Which of the following are paramagnetic?
A. \([\text{NICI}_4]^{2-}\)
B. \(\text{Ni(CO)}_4\)
C. \([\text{NICN}_4]^{2-}\)
D. \([\text{NI(H)}_2\text{O)}_6]^{2+}\)
E. \(\text{Ni(PPh}_3\text{)}_4\)
Choose the correct answer from the options given below:
A. \([\text{NICI}_4]^{2-}\)
B. \(\text{Ni(CO)}_4\)
C. \([\text{NICN}_4]^{2-}\)
D. \([\text{NI(H)}_2\text{O)}_6]^{2+}\)
E. \(\text{Ni(PPh}_3\text{)}_4\)
Choose the correct answer from the options given below:
Solution
Solution
Year: 2024
Topic: Coordination Compounds
11.
Statement I:
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below