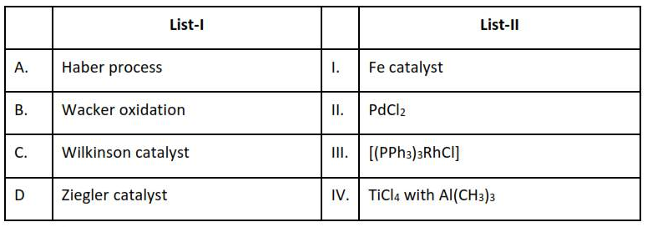
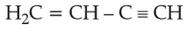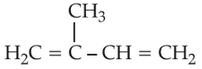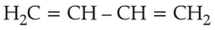Home >> Topics >> The d-and f-Block Elements - Questions Not Attempted
Unattempted Questions
Questions Available: 20
Year: 2023
Topic: The d-and f-Block Elements
1.
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
Solution
Solution
Year: 2025
Topic: The d-and f-Block Elements
3.
Given below are two statements :
Statement I : Ferromagnetism is considered as an extreme form of paramagnetism.
Statement II : The number of unpaired electrons in a \(Cr^{2+}\) ion (Z=24) is the same as that of a \(Nd^{3+}\) ion (Z= 60).
In the light of the above statements, choose the correct answer from the options given below :
Statement I : Ferromagnetism is considered as an extreme form of paramagnetism.
Statement II : The number of unpaired electrons in a \(Cr^{2+}\) ion (Z=24) is the same as that of a \(Nd^{3+}\) ion (Z= 60).
In the light of the above statements, choose the correct answer from the options given below :
Solution
Year: 2024
Topic: The d-and f-Block Elements
4.
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
Solution
Solution
Solution
Solution
Year: 2023
Topic: The d-and f-Block Elements
8.
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below: