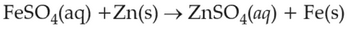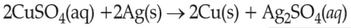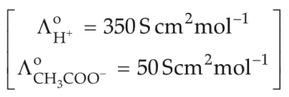Home >> Topics >> Electrochemistry
Unattempted Questions
Questions Available: 17
Year: 2025
Topic: Electrochemistry
1.
If the molar conductivity (\(\Lambda_m\)) of a 0.050 mol L-1 solution of a monobasic weak acid is 90 S cm2 mol-1, its extent (degree) of dissociation will be
[Assume \(\Lambda_+^\circ\, =\, 349.6 \,S\, cm^2\, mol^{-1}\) and \(\Lambda_-^2\, =\, 50.4\, S\, cm^2\, mol^{-1}\)]
[Assume \(\Lambda_+^\circ\, =\, 349.6 \,S\, cm^2\, mol^{-1}\) and \(\Lambda_-^2\, =\, 50.4\, S\, cm^2\, mol^{-1}\)]
Solution
Solution
Solution
Solution
Solution
Year: 2022
Topic: Electrochemistry
6.
At 298 K, the standard electrode potentials of
\(\displaystyle \frac{Cu^{2+}}{Cu}\), \(\displaystyle \frac{Zn^{2+}}{Zn}\), \(\displaystyle \frac{Fe^{2+}}{Fe}\), and \(\displaystyle \frac{Ag^{+}}{Ag}\) are \(0.34\, \text{V}\), \(-0.76\, \text{V}\), \(-0.44\, \text{ V}\) and \(0.80\, \text{V}\), respectively.
On the basis of standard electrode potential, predict which of the following reaction cannot occur?
\(\displaystyle \frac{Cu^{2+}}{Cu}\), \(\displaystyle \frac{Zn^{2+}}{Zn}\), \(\displaystyle \frac{Fe^{2+}}{Fe}\), and \(\displaystyle \frac{Ag^{+}}{Ag}\) are \(0.34\, \text{V}\), \(-0.76\, \text{V}\), \(-0.44\, \text{ V}\) and \(0.80\, \text{V}\), respectively.
On the basis of standard electrode potential, predict which of the following reaction cannot occur?
Solution
Solution
Solution
Solution
Solution
Solution
Solution
Year: 2019
Topic: Electrochemistry
13.
For the cell reaction
2Fe3+(aq) + 2I–(aq) \(\rightarrow\) 2Fe2+(aq) + I2 (aq)
\(\text{E} ^\oplus_\text{cell}\) = 0.24 V at 298 K. The standard Gibbs energy cell (\(\Delta_r \text{G}^\ominus\)) of the cell reaction is
[Given that Faraday constant F = 96500 C mol–1]
2Fe3+(aq) + 2I–(aq) \(\rightarrow\) 2Fe2+(aq) + I2 (aq)
\(\text{E} ^\oplus_\text{cell}\) = 0.24 V at 298 K. The standard Gibbs energy cell (\(\Delta_r \text{G}^\ominus\)) of the cell reaction is
[Given that Faraday constant F = 96500 C mol–1]
Solution
Solution
Solution
Year: 2017
Topic: Electrochemistry
16.
In the electrochemical cell
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)