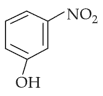
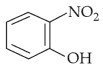
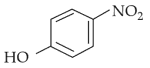
Home >> Topics >> Structure of Atom - Questions Not Attempted
Unattempted Questions
Questions Available: 19
Solution
Solution
Solution
Solution
Solution
Solution
Year: 2024
Topic: Structure of Atom
7.
Given below are two statements:
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
Solution
Solution
Solution
Year: 2023
Topic: Structure of Atom
10.
Select the correct Statements from the following:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below: