Home >> NEET - 2016
Welcome and Shine in NEET with Shine NEET SmartPrep!.
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Your Full Test Performance Summary
Questions Available: 180
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
1.
Match the corresponding entries of Column 1 with Column 2. [Where m is the magnification produced by the mirror]
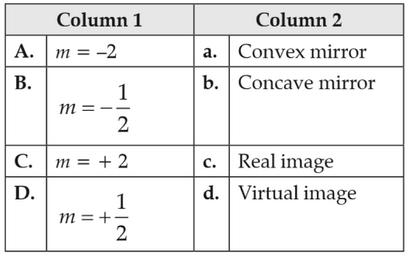

2.
What is the minimum velocity with which a body of mass m must enter a vertical loop of radius R so that can complete the loop?
3.
From a disc of radius R and mass M, a circular hole of diameter R, whose rim passes through the centre is cut. What is the moment of inertia of the remaining part of the disc about a perpendicular axis, passing through the centre?
4.
If the magnitude of sum of two vectors is equal to the magnitude of difference of the two vectors, the angle between these vectors is:
5.
When an \(\alpha\)-particle of mass m moving with velocity v bombards on a heavy nucleus of charge Ze, its distance of closest approach from the nucleus depends on m as
6.
A potentiometer wire is 100 cm long and a constant potential difference
is maintained across it. Two cells are connected in series first to
support one another and then in opposite direction. The balance points
are obtained at 50 cm and 10 cm from the positive end of the wire in the
two cases. The ratio of emf's is
7.
A particle of mass 10 g moves along a circle of radius 6.4 cm with a constant tangential acceleration. What is the magnitude of this acceleration if the kinetic energy of the particle becomes equal to 8 × 10−4J by the end of the second revolution after the beginning of the motion ?
8.
A body of mass 1 kg begins to move under the action of time dependent force \(\vec{F}\) = \(\left(2t\hat{i} + 3t^2\hat{j}\right)\) N , where \(\hat{i}\) and \(\hat{j}\) are unit vectors along x and y axis.What power will developed by the force at the time (t) ?
9.
The angle of incidence for a ray of light at a refracting surface of a prismis \(45^\circ\). The angle of prism is \(60^\circ\). If the ray suffers minimum deviation through the prism, the
angle of minimum deviation and refractive index of the material of the prism respectively, are
10.
At what height from the surface of earth the gravitation potential and the value of g are \(-\, 5.4 × 10^7J\, \text{Kg}^{−2}\) and \(6.0\,\text{ms}^{−2}\) respectively? Take the radius of earth as 6400 km.
11.
The ratio of escape velocity at earth (\(v_e\)) to the escape velocity at a planet (\(v_p\)) whose radius and mean density are twice as that of earth is
12.
A astronomical telescope has objective and eyepiece of focal lengths 40 cm and 4 cm respectively. To view an object 200 cmaway from the objective, the lenses must be separated by a distance
13.
The intensity at the maximum in a Young’s double slit experiment is \(I_0\). Distance between two slits is \(d\, =\, 5\lambda\), where \(\lambda\) is the wavelength of light used in the experiment. What will be the intensity in front of one of the slits on the screen placed at a distance D = 10 d?
14.
In a diffraction pattern due to a single slit of width a, the first minimum is observed at an angle 30° when light of wavelength 5000 Å is incident on the slit. The first secondary maximum is observed at an angle of
15.
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half.
16.
A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant.The power required is (Take, 1 cal =4.2Joules)
17.
If the velocity of a particle is \(\text{v}\, =\, \text{At}\, +\, \text{Bt}^2\), where A and B are constants, then the distance travelled by it between \(1\,s\) and \(2\,s\) is
18.
A disc and a sphere of same radius but different masses roll off on two inclined planes of the same altitude and length. Which one of the two objects gets to the bottom of the plane first?
19.
A uniform circular disc of radius 50 cm at rest is free to turn about an axis which is perpendicular to its plane and passes through its centre. It is subjected to a torque which produces a constant angular acceleration of 2.0 rad s−2. Its net acceleration in s−2 at the end of 2.0 S is approximately
20.
The molecules of a given mass of a gas have r.m.s. velocity of \(200\, \text{ms}^{−1}\) at \(27^\circ \text{C}\) and \(1.0 \times 10^5 \text{Nm}^{−2}\) pressure. When the temperature and pressure of the gas are respectively, \(127^ \circ \text{C}\) and \(0.05 \times 10^5 \text{Nm}^{ −2}\) , the rms velocity of its molecules in \(\text{ms}^{ −1}\) is
21.
An electron of mass m and a photon have same energy E. The ratio ofde-Broglie wavelengths associated with them is(c being velocity of light)
22.
When a metallic surface is illuminated with radiation of wavelength \(\lambda\), the stopping potential is \(\text{V}\). If the same surface is illuminated with radiation of wavelength \(2\lambda\), the stopping potential is \(\displaystyle \frac{V}{4}\).The threshold wavelength for the metallic surface is
23.
Two identical charged spheres suspended from a common point by two mass less strings of lengths l, are initially at a distance d (d < < l) apart because of their mutual repulsion. The charges begin to leak from both the spheres at a constant rate. As a result, the spheres approach each other witha velocity \(v\). Then, \(v\) varies as a function of the distance \(x\) between the sphere, as
24.
Coefficient of linear expansion of brass and steel rods are \(\alpha_1\) and \(\alpha_2\). Length of brass and steel rods are \(l_1\) and \(l_2\) respectively. If \(\left(l_2 − l_1\right)\) is maintained same at all temperatures, which one of the following relations holds good?
25.
A piece of ice falls from a height h so that it melts completely. Only one-quarter of the heat produced is absorbed by the ice and all energy of ice gets converted into heat during its fall. The value of \(h\) is [Latent heat of ice is \(3.4 × 10^5 J/kg\) and \(g = 10 N/kg\)
26.
A black body is at a temperature of \(5760\, \text{K}\). The energy of radiation emitted by the body at wavelength \(250\, \text{nm}\) is \(\text{U}_1\),at wavelength \(500\, \text{nm}\) is \(\text{U}_2\) and that at \(1000\, \text{nm}\) is \(\text{U}_3\) Wien's constant, \(\text{b}\, =\, 2.88 \times 10^6\, \text{nmK}\). Which of the following is correct?
27.
Two non-mixing liquids of densities \(p\) and \(np \left(n > 1\right)\) are put in a container. The height of each liquid is \(\text{"A"}\). A solid cylinder of length \(\text{L}\) and density \(\text{d}\) is put in this container. The cylinder floats with its axis vertical and length \(pL \left(p < 1\right)\) in the denser liquid. The density \(d\) is equal to
28.
To get output 1 for the following circuit, the correct choice for the inputis
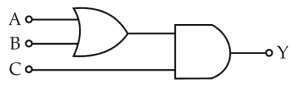

29.
A npn transistor is connected in common emitter configuration in a given amplifier. A load resistance of \(800\, \Omega\) is connected in the collector circuit and the voltage drop across it is \(0.8\, \text{V}\). If the current amplification factor is \(0.96\) and the input resistance of the circuit is \(192\, \Omega\), the voltage gain and the power gain of the amplifier will respectively be
30.
Consider the junction diode as ideal. The value of current flowing through AB is
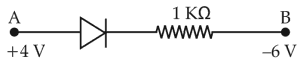

31.
A particle moves so that its position vector is given by \(\vec{r}\, =\, cos \,\omega \,t\, \hat{x}\, +\, sin\, \omega\,t\,\hat{y}\), where \(\omega\) is a constant. Which of the following is true ?
32.
An inductor 20 mH, a capacitor 50 μF and a resistor 40 Ω are connected in series across a source of emf
\(V\, =\, 10\,sin\,340\,t\). The power loss in A.C. circuit is
\(V\, =\, 10\,sin\,340\,t\). The power loss in A.C. circuit is
33.
A small signal voltage V (t) = V0 sin ωt is applied across an ideal capacitor C
34.
A long solenoid has 1000 turns. When a current of 4 A flows through it, the magnetic flux linked with each turn of the solenoid is 4 × 10 −3 Wb. The self-inductance of the solenoid is
35.
A siren emitting a sound of frequency \(800\, Hz\) moves away from an observer towards a cliff at a speed of \(15\, \text{ms}^{−1}\). Then, the frequency of sound that the observer hears in the echo reflected from the cliff is (Take velocity of sound in air = \(330\,\text{ms}^{−1}\))
36.
An air column, closed at one end and open at the other, resonates with a tuning fork when the smallest length of the column is \(50\, \text{Cm}\). The next larger length of the column resonating with the same tuning fork is
37.
A uniform rope of length \(L\) and mass \(m_1\) hangs vertically from a rigid support. A block of mass \(m_2\) is attached to the free end of the rope. A transverse pulse of wavelength \(λ_1\) is produced at the lower end of the rope. The wavelength of the pulse when it reaches the top of the rope is \(λ_2\).The ratio \(\displaystyle \frac{λ_1}{λ_2}\) is
38.
A capacitor of 2 μF is charged as shown in the diagram. When the switch S is turned to position 2, the percentage of its stored energy dissipated is
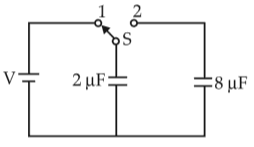

39.
A car is negotiating a curved road of radius R. The road is banked at anangle θ. The coefficient of friction between the tyres of the car and the road is μs. The maximum safe velocity on this road is
40.
The magnetic susceptibility is negative for
41.
Given the value of Rydberg constant is \(10\,\text{m}^{-1}\), the wave number of the last line of the Balmer series in hydrogen spectrum will be
42.
The charge flowing through a resistance R varies with time t as \(\text{Q}\, =\, at − bt^2\), where a and b are positive constants. The total heat produced in R is
43.
Out of the following options which one can be used to produce a propagating electromagnetic wave?
44.
A long straight wire of radius a carries a steady current I. The current is uniformly distributed over its cross-section. The ratio of the magnetic fields B and B', at radial distances \(\displaystyle\frac{a}{2}\) and \(2a\) respectively, from the axis of the wire is
45.
A square loop ABCD carrying a current is placed near and coplanar with a long straight conductor XY carrying a current I, the net force on the loop will be
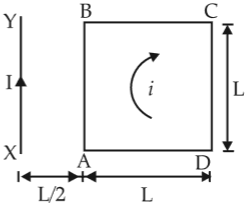

46.
Match items of Column I with the items of Column II and assign the correct code.
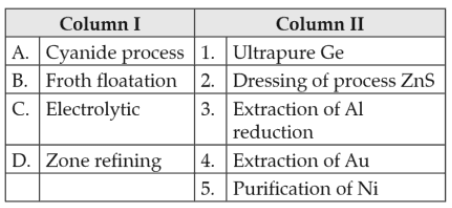
Codes

Codes
47.
Which is the correct statement for the given acids ?
48.
Fog is a colloidal solution of
49.
Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules ?
50.
MY and NY3, two nearly insoluble salts have the same Ksp values of 6.2 × 10-13 at room temperature. Which statement would be true in regard to MY and NY3 ?
51.
Which of the following statements about hydrogen is incorrect ?
52.
The correct statement regarding the basicity of arylamines is
53.
When copper is heated with conc. HNO3 it produces
54.
For the following reactions,
(i) CH3CH2CH2Br + KOH \(\rightarrow\) CH3CH = CH2 + KBr + H2O
(ii)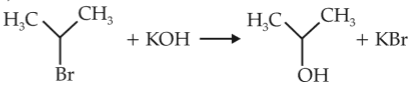
(iii)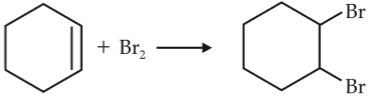
Which of the following statement is correct ?
(i) CH3CH2CH2Br + KOH \(\rightarrow\) CH3CH = CH2 + KBr + H2O
(ii)

(iii)

Which of the following statement is correct ?
55.
Two electrons occupying the same orbital are distinguished by
56.
he reaction
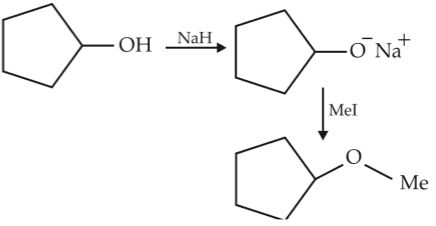
can be classified as

can be classified as
57.
The electronic configurations of Eu (Atomic no. 63), Gd (Atomic no. 64) and Tb (Atomic no. 65) are
58.
At 100°C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
59.
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is
60.
Which one of the following characteristics is associated with adsorption ?
61.
Match the compounds given in column I with the hybridisation and shape given in column II and mark the correct option.
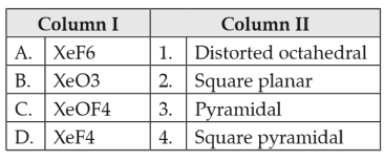

62.
The correct statement regarding a carbonyl compound with a hydrogen atom on its alpha-carbon, is
63.
In a protein molecule various amino acids are linked together by
64.
Which of the following is an analgesic ?
65.
The pair of electron in the given carbanion, CH3C≡C–, is present in which orbitals ?
66.
Consider the molecules CH4, NH3 and H2O. Which of the given statements is false ?
67.
Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7 solution ?
68.
The correct thermodynamic conditions for the spontaneous reaction at all temperatures is
69.
Natural rubber has
70.
In which of the following options the order of arrangement does not agree with the variation of property indicated against it ?
71.
Which of the following reagents would distinguish cis - cyclopenta - 1 , 2 - diol from the trans - isomer ?
72.
The product obtained as a result of a reaction of nitrogen with CaC2 is
73.
Equal moles of hydrogen and oxygen gases are placed in container with a pin - hole through which both can escape . What fraction of the oxygen escapes in the time required for one - half of the hydrogen to escape ?
74.
Lithium has a bcc structure . Its density is 530 kg m-3 and its atomic mass is 6.94 g mol-1 . Calculate the edge length of a unit cell of lithium metal.
( NA = 6.02 × 1023 mol-1 ).
( NA = 6.02 × 1023 mol-1 ).
75.
Which of the following statements about the composition of the vapour over an ideal 1 : 1 molar mixture of benzene and toluene is correct ? Assume that the temperature is constant at 25 ° C.
( Given , vapour pressure data at 25 ° C , benzene = 12.8 kPa , toluene = 3.85 kPa )
( Given , vapour pressure data at 25 ° C , benzene = 12.8 kPa , toluene = 3.85 kPa )
76.
Which of the following has longest C – O bond length ? ( Free C - O bond length in CO is 1.128 Å . )
77.
Among the following , the correct order of acidity is
78.
In the reaction,
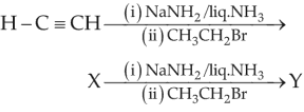
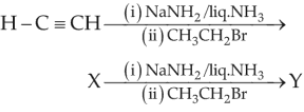
79.
Consider the nitration of benzene using mixed conc . H2SO4 and HNO3 . If a large amount of KHSO4 is added to the mixture , the rate of nitration will be
80.
The product formed by the reaction of an aldehyde with a primary amine is
81.
The pressure ofH2 required to make the potential of H<2/sub> - electrode zero in pure water at 298 K is
82.
The correct statement regarding RNA and DNA , respectively is
83.
Which one given below is a non - reducing sugar ?
84.
Consider the following liquid - vapour equilibrium
Liquid \(\rightleftharpoons\) Vapour
Which of the following relations is correct ?
Liquid \(\rightleftharpoons\) Vapour
Which of the following relations is correct ?
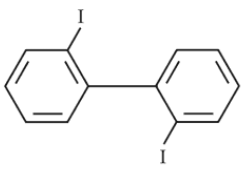
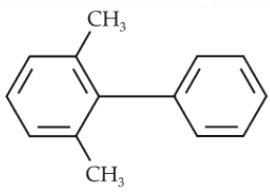
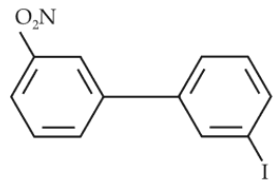
85.
Which of the following biphenyls is optically active ?
86.
Which of the following statements is false ?
87.
The ionic radii of A + and B- ions are 0.98 × 10-10 m and 1.81 × 10-10 m . The coordination number of each ion in AB is
88.
The rate of a first - order reaction is 0.04 mol L-1S-1 at 10 sec and 0.03 mol L-1S-1 at 20 sec after initiation of the reaction . The half - life period of the reaction is
89.
The addition of a catalyst during a chemical reaction alters which of the following quantities ?
90.
Predict the correct order among the following .
91.
Mitochondria and chloroplast are
I. semi-autonomous organelles
II. formed by the division of pre existing organelles and they contain DNA but lack protein synthesising machinery
Which one of the following options is correct?
I. semi-autonomous organelles
II. formed by the division of pre existing organelles and they contain DNA but lack protein synthesising machinery
Which one of the following options is correct?
92.
Chrysophytes, euglenoids, dinoflagellates and slime moulds are included in the kingdom
93.
A tall true breeding garden pea plant is crossed with a dwarf true breeding garden pea plant. When the F1 plants were selfed, the resulting genotypes were in the ratio of
94.
Depletion of which gas in the atmosphere can lead to an increased incidence of skin cancers
95.
In the stomach, gastric acid is secreted by the
96.
Which of following is wrongly matched in the given table ?
97.
Which of the following is not a stem modification ?
98.
Select the incorrect statement
99.
One of the major components of cell wall of most fungi is
100.
Which of the following statements is not correct ?
101.
Which of the following is not a characteristic feature during mitosis in somatic cells ?
102.
In context of amniocentesis, which of the following statement is incorrect ?
103.
The Taq polymerase enzyme is obtained from
104.
The standard petal of a papilionaceous corolla is also called
105.
Which of the following characteristic features always holds true for the corresponding group of animals ?
106.
Changes in GnRH pulse frequency in females is controlled by circulating levels of
107.
Microtubules are the constituents of
108.
Photosensitive compound in human eye is made up of
109.
The primitive prokaryotes responsible for the production of biogas from the dung of ruminant animals, include the
110.
Identify the correct statement on ‘inhibin’.
111.
It is much easier for a small animal to run uphill than for a large animal, because
112.
Which one of the following is a characteristic feature of cropland ecosystem ?
113.
Tricarpellary, syncarpous gynoecium is found in the flowers of
114.
In which of the following, all the three are macronutrients ?
115.
Reduction in pH of blood will
116.
Lack of relaxation between successive stimuli in sustained muscle contraction is known as
117.
Which one of the following statements is wrong ?
118.
Which of the following is a restriction endonuclease?
119.
Which of the following would appear as the pioneer organisms on bare rocks ?
120.
Water vapour comes out from the plant leaf through the stomatal opening. Through the same stomatal opening carbon dioxide diffuses into the plant during photosynthesis. Reason out the above statements using the following options.
121.
Cotyledon of maize grain is called
122.
Which of the following guards the opening of hepatopancreatic duct into the duodenum ?
123.
In the mammals, which blood vessel would normally carry largest amount of urea ?
124.
The term ecosystem was coined by
125.
Which of the following is required as inducer(s) for the expression of lac operon ?
126.
When does the growth rate of a population following the logistic modal equals zero ? The logistic model is given as
\(\displaystyle \frac{\text{dN}}{\text{dt}}\) = \(\displaystyle \text{rN} \left(\frac{\text{1-N}}{\text{K}}\right)\)
\(\displaystyle \frac{\text{dN}}{\text{dt}}\) = \(\displaystyle \text{rN} \left(\frac{\text{1-N}}{\text{K}}\right)\)
127.
Which one of the following statements is not true ?
128.
In bryophytes and pteridophytes, transport of male gametes requires
129.
Which one of the following cell organelles is enclosed by a single membrane?
130.
Analogous structures are a result of
131.
Which one of the following statements is wrong ?
132.
Proximal end of the filament of stamen is attached to the
133.
Which of the following is not required for any of the techniques of DNA fingerprinting available at present ?
134.
Which one of the following characteristics is not shared by birds and mammals ?
135.
The amino acid, tryptophan is the precursor for the synthesis of
136.
Joint Forest Management Concept was introduced in India during
137.
A complex of ribosomes attached to a single strand of RNA is known as
138.
Which of the following features is not present in the phylum - Arthropoda?
139.
Asthma may be attributed to
140.
Pick out the correct statements
I. Haemophilia is a sex linked recessive disease
II . Down's syndrome is due to aneuploidy
III . Phenylketonuria is an autosomal recessive gene disorder
IV . Sickle cell anaemia is an X linked recessive gene disorder
I. Haemophilia is a sex linked recessive disease
II . Down's syndrome is due to aneuploidy
III . Phenylketonuria is an autosomal recessive gene disorder
IV . Sickle cell anaemia is an X linked recessive gene disorder
141.
The two polypeptides of human insulin are linked together by
142.
The coconut water from tender coconut represents
143.
Which of the following is not a feature of the plasmids ?
144.
Which is the National Aquatic Animal of India ?
145.
The Avena curvature is used for bioassay of
146.
Which of the following is the most important cause of animals and plants being driven to extinction?
147.
Which of the following approaches does not give the defined action of contraceptive ?
Microbe: Product
Microbe: Product
148.
In a test cross involving F1 dihybrid flies, more parental-type offspring were produced than the recombinant type offspring. This indicates
149.
A typical fat molecule is made up of
150.
Match the terms in Column I with their description in Column II and choose the correct option.


151.
Which of the following features is not present in Periplaneta americana ?
152.
Water soluble pigments found in plant cell vacuoles are
153.
A cell at telophase stage is observed by a student in a plant brought from the field. He tells his teacher that this cell is not like other cells at telophase stage. There is no formation of cell plate and thus the cell is containing more number of chromosomes as compared to other dividing cells. This would result in
154.
A plant in your garden avoids photorespiratory losses, has improved water use efficiency, shows high rates of photosynthesis at high temperatures and has improved efficiency of nitrogen utilisation. In which of the following physiological groups would you assign this plant ?
155.
In higher vertebrates, the immune system can distinguish self-cells and non-self. If this property is lost due to genetic abnormality and it attacks self-cells, then it leads to
156.
Emerson's enhancement effect and red drop have been instrumental in the discovery of
157.
Select the correct statement
158.
Blood pressure in the pulmonary artery is
159.
Which of the following structures is homologous to the wing of a bird ?
160.
Seed formation without fertilisation in flowering plants involves the process of
161.
Name the chronic respiratory disorder caused mainly by cigarette smoking
162.
Spindle fibres attach on to
163.
Stems modified into flat green organs performing the functions of leaves are known as
164.
In a chloroplast the highest number of protons are found in
165.
Nomenclature is governed by certain universal rules. Which one of the following is contrary to the rules of nomenclature ?
166.
In meiosis crossing over is initiated at
167.
Antivenom injection contains preformed antibodies while polio drops that are administered into the body contain
168.
Which of the following most appropriately describes haemophilia ?
169.
Which part of the tobacco plant is infected by Meloidogyne incognita ?
170.
Which of the following statements is wrong for viroids ?
171.
Which of the following statements is not true for cancer cells in relation to mutations ?
172.
Which type of tissue correctly matches with its location ?
173.
Which of the following pairs of hormones are not antagonistic (having opposite effects) to each other ?
174.
Specialised epidermal cells surrounding the guard cells are called
175.
Fertilisation in humans is practically feasible only if
176.
Which one of the following is the start codon ?
177.
A river with an inflow of domestic sewage rich in organic waste may result in
178.
Following are the two statements regarding the origin of life
I. The earliest organisms that appeared on the earth were non-green and presumably anaerobes
II. The first autotrophic organisms were the chemoautotrophs that never released oxygen
Of the above statements which one of the following options is correct ?
I. The earliest organisms that appeared on the earth were non-green and presumably anaerobes
II. The first autotrophic organisms were the chemoautotrophs that never released oxygen
Of the above statements which one of the following options is correct ?
179.
A system of rotating crops with legume or grass pasture to improve soil structure and fertility is called
180.
Gause’s principle of competitive exclusion states that