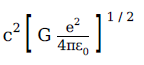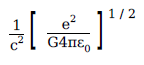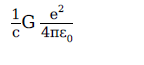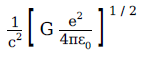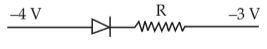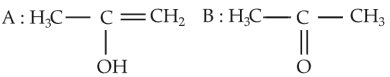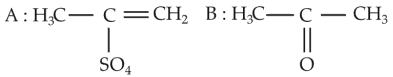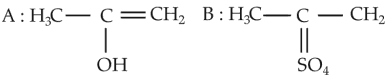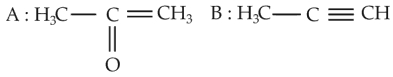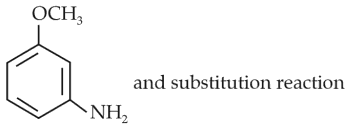Home >> NEET - 2017
Welcome and Shine in NEET with Shine NEET SmartPrep!.
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Your Full Test Performance Summary
Questions Available: 180
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
1.
A beam of light from a source L is incident normally on a plane mirror fixed at a certain distance x from the source. The beam is reflected back as a spot on a scale placed just above the source L. When the mirror is rotated through a small angle "q", the spot of the light is found to move through a distance y on the scale. The angle \(\theta\) is given by
2.
The ratio of resolving powers of an optical microscope for two wavelengths \(\lambda_1 = 4000\,\mathring{A}\) and \(\lambda_2 = 6000\, \mathring{A}\) is
3.
Suppose the charge of a proton and an electron differ slightly. One of them is \(−e\) and the other is \(\left(e + \Delta e\right)\). If the net of electrostatic force and gravitational force between two hydrogen atoms placed at a distance d (much greater than atomic size) apart is zero, then \(\Delta e\) is of the order [Given mass of hydrogen, \(m_h =1.67 × 10^{−27} kg\)]
4.
One end of string of length \(l\) is connected to a particle of mass \('m'\) and the other end is connected to a small peg on a smooth horizontal table.If the particle moves in circle with speed 'V' , the net force on the particle (directed towards center) will be (T represents the tension in the string)
5.
Consider a drop of rain water having mass 1 g falling from a height of 1km. It hits the ground with a speed of 50ms−1. Take 'g' constant with a value 10ms−2.The work done by the (i) gravitational force and the (ii) resistive force of air is
6.
A thin prism having refracting angle \(10^\circ\) is made of glass of refractive index 1.42. This prism is combined with another thin prismof glass of refractive index 1.7. This combination produces dispersion without deviation. The refracting angle of second prism should be
7.
The acceleration due to gravity at a height 1 Km above the earth is the same as at a depth "d" below the surface of earth. Then
8.
Two astronauts are floating in gravitational free space after having lost contact with their spaceship. The two will
9.
Young’s double slit experiment is first performed in air and then in a medium other than air. It is found that 8th bright fringe in the medium lies where 5th dark fringe lies in air. The refractive index of the medium is nearly
10.
A physical quantity of the dimensions of length that can be formed out of c, G and \(\displaystyle \frac{e^2}{4\pi \, \epsilon_0}\) is
[c is velocity of light, G is the universal constant of gravitation and e is charge]
[c is velocity of light, G is the universal constant of gravitation and e is charge]
11.
Two polaroids \(\text{P}_1\) and \( \text{P}_ 2\) are placed with their axis perpendicular to each other. Unpolarised light \(\text{I}_0\) is incident on \(\text{P}_1\) . A third polaroid \(\text{P}_3\) is kept in between \(\text{P}_1\) and \(\text{P}_2\) such that its axis makes an angle 45° with that of \(\text{P}_1\). The intensity of transmitted light through \(\text{P}_2\) is
12.
Thermodynamic processes are indicated in the following diagram
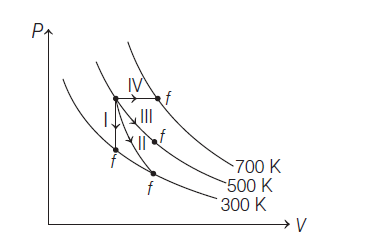
Match the following:

Match the following:

13.
A rope is wound around a hollow cylinder of mass 3 kg and radius 40 cm. What is the angular acceleration of the cylinder if the rope is pulled with a force of 30 N?
14.
Two discs of same moment of inertia rotating about their regular axis passing through centre and perpendicular to the plane of disc with angular velocities ω1 and ω2.They are brought into contact face to face coinciding the axis of rotation. The expression for loss of energy during this process is
15.
A Carnot engine having an efficiency of 110 as heat engine, is used as a refrigerator. If the workdone on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
16.
Which of the following statements are correct?
(1) Centre of mass of a body always coincides with the centre of gravity of the body.
(2) Centre of mass of a body is the point at which the total gravitational torque on the body is zero.
(3) A couple on a body produces both transnational and rotational motion in a body.
(4) Mechanical advantage greater than one means that small effort can be used to lift a large load.
(1) Centre of mass of a body always coincides with the centre of gravity of the body.
(2) Centre of mass of a body is the point at which the total gravitational torque on the body is zero.
(3) A couple on a body produces both transnational and rotational motion in a body.
(4) Mechanical advantage greater than one means that small effort can be used to lift a large load.
17.
Preeti reached the metro station and found that the escalator was not working. She walked up the stationary escalator in time \(t_1\). On other days, if she remains stationary on the moving escalator, then the escalator takes her up in time \(t_2\). The time taken by her to walk up on the moving escalator will be
18.
A gas mixture consists of 2 moles of \(O_2\) and 4 moles of \(A_r\) at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
19.
The de-Broglie wavelength of a neutron in thermal equilibrium with heavy water at a temperature T (kelvin) and mass m, is
20.
The photoelectric threshold wavelength of silver is \(3250 \times 10^{−10}\,m\). The velocity of the electron ejected from a silver surface by ultraviolet light of wavelength \(2536 \times 10^{−10}\,m\) is
[Given: \(h \,=\, 4.14 \times 10^{−15}\, \text{eVs}\) and \(c \,=\, 3 \times 10^8\,ms^{−1}\)]
[Given: \(h \,=\, 4.14 \times 10^{−15}\, \text{eVs}\) and \(c \,=\, 3 \times 10^8\,ms^{−1}\)]
21.
Two rods \(A\) and \(B\) of different materials are welded together as shown infigure. Their thermal conductivities are \(K_1\) and \(K_2\) The thermal conductivity of the composite rod will be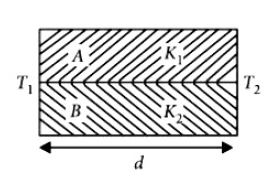

22.
A spherical black body with a radius of \(12\, \text{cm}\) radiates \(450\, \text{watt}\) power at \(500\, \text{K}\). If the radius were halved and the temperature doubled, the power radiated in watt would be
23.
The bulk modulus of a spherical object is \('B'\). If it is subjected to uniform pressure \('p'\) the fractional decrease in radius is
24.
In a common emitter transistor amplifier the audio signal voltage across the collector is 3 V. The resistance of collector is 3 KΩ. If current gain is 100 and the base resistance is 2 KΩ, the voltage and power gain of the amplifier is
25.
The given electrical network is equivalent to
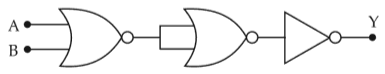

26.
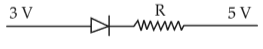
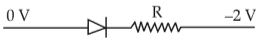
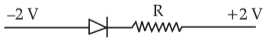
Which one of the following represents forward bias diode?
27.
The x and y coordinates of the particle at any time are x = 5t − 2\(t^2 \) and y = 10t respectively, where x and y are in meters and t in seconds. The acceleration of the particle at t = 2 s
28.
A long solenoid of diameter 0.1 m has 2 × 104 turns per metre. At the centre of the solenoid, a coil of 100 turns and radius 0.01 m is placed with its axis coinciding with the solenoid axis. The current in the solenoid reduces at a constant rate to 0 A from 4 A in 0.05 s. If the resistance of the coil is 10π2Ω, the total charge flowing through the coil during this time is
29.
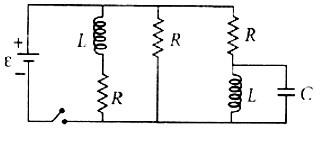
Figure shows a circuit that contains three identical resistors with resistance R = 9.0Ω each, two identical inductors with inductance L =2.0 mH each, and an ideal battery with emf ε = 18V . The current i through the battery just after the switch closed is

30.
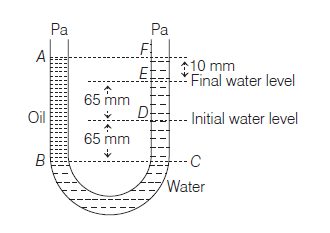
A U tube with both ends open to the atmosphere, is partially filled with water. Oil, which is immiscible with water, is poured into one side until it stands at a distance of 10mm above the water level on the other side. Mean while the water rises by 65 mm from its original level (see diagram). The density ofthe oil is

31.
A spring of force constant k is cut into lengths of ratio \(1 : 2 : 3\). They are connected in series and the new force constant is \(k'\).Then they are connected in parallel and force constant is \(k''\).Then \(k' : k''\) is
32.
A particle executes linear simple harmonic motion with an amplitude of \(3\, \text{cm}\). When the particle is at \(2\, \text{cm}\) from the mean position, the magnitude of its velocity is equal to that of its acceleration. Then its time period in seconds is
33.
The ratio of wavelengths of the last line of Balmer series and the last line of Lyman series is
34.
Radioactive material A has decay constant \(8 \lambda\) and material B has decay constant \(\lambda\). Initially, they have same number of nuclei. After what time, the ratio of number of nuclei of material B to that A will be \(\frac{1}{e}\) ?
35.
The two nearest harmonics of a tube closed at one end and open at other end are \(220\, Hz\) and \(260\, Hz\). What is the fundamental frequency of the system?
36.
Two cars moving in opposite directions approach each other with speed of \(22\,ms^{−1}\) and \(16.5\,ms^{−1}\) respectively. The driver of the first car blows a horn having a frequency \(400\, Hz\). The frequency heard by the driver of the second car is [velocity of sound is \(340\,ms^{−1}\)]
37.
A capacitor is charged by a battery. The battery is removed and another identical uncharged capacitor is connected in parallel. The total electrostatic energy of resulting system
38.
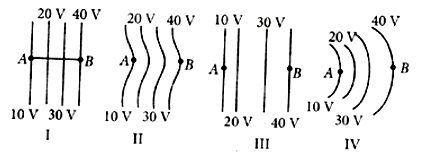
The diagrams below show regions of equipotential. A positive charge is moved from A to B in each diagram.

39.
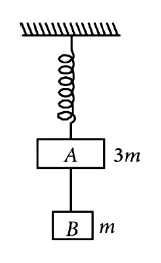
Two blocks A and B of masses 3m and m respectively are connected by a mass-less and in extensible string. The whole system is suspended by a mass-less spring as shown in figure. The magnitudes of acceleration of A and B immediately after the string is cut are, respectively

40.
If \(θ_1\) and \(θ_2\) be the apparent angles of dip observed in two vertical planes at right angles to each other, then the true angle of dip \(\theta\) is given by
41.
A 250 turn rectangular coil of length 2.1 cm and width 1.25 cm carries a current of 85 µA and subjected to a magnetic field of strength 0.85 T. Work done for rotating the coil by 180° against the torque is
42.
The resistance of a wire is 'R' ohm. If it is melted and stretched to 'n'times its original length, its new resistance will be
43.
A potentiometer is an accurate and versatile device to make electrical
measurements of EMF because the method involves
44.
In an electromagnetic wave in free space the root mean square value of the electric field is \(E_{rms}\,=\, 6V m^{−1}\).The peak value of the magnetic field is
45.
An arrangement of three parallel straight wires placed perpendicular to plane of paper carrying same current I along the same direction as shown in figure. Magnitude of force per unit length on the middle wire ‘B’ is given by
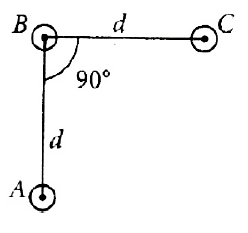

46.
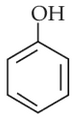
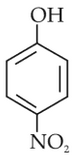
The most suitable method of separation of 1 : 1 mixture of ortho and para-nitrophenols is
47.
Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
48.
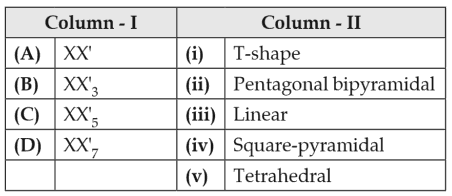
Match the interhalogen compounds of column I with the geometry in column II and assign the correct code.

49.
Which one of the following pairs of species have the same bond order ?
50.
HgCl2 and I 2 both when dissolved in water containing I- ions, the pair ofspecies formed is
51.
The equilibrium constants of the following are:
N2 + 3H2 \(\rightleftharpoons\) 2NH3 ------ K1
N2 + O2 \(\rightleftharpoons\) 2NO ------ K2
H2 + \(\frac{\text{1}}{\text{2}}\)O2 \(\rightarrow \) H2O ------ K3
The equilibrium constant (K) of the reaction:
2NH3 + \(\frac{\text{5}}{\text{2}}\)O2 \(\rightleftharpoons\) 2NO + 3H2O, will be
N2 + 3H2 \(\rightleftharpoons\) 2NH3 ------ K1
N2 + O2 \(\rightleftharpoons\) 2NO ------ K2
H2 + \(\frac{\text{1}}{\text{2}}\)O2 \(\rightarrow \) H2O ------ K3
The equilibrium constant (K) of the reaction:
2NH3 + \(\frac{\text{5}}{\text{2}}\)O2 \(\rightleftharpoons\) 2NO + 3H2O, will be
52.
The heating of phenyl-methyl ethers with HI produces
53.
Predict the correct intermediate and product in the following reaction
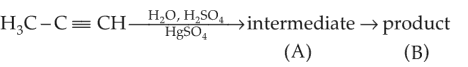
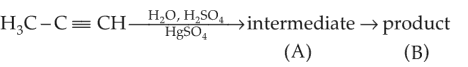
54.
Which of the following reactions is appropriate for converting acetamide to methanamine ?
55.
The IUPAC name of the compound is
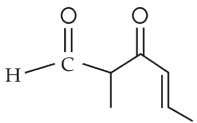

56.
Which of the following is a sink for CO?
57.
Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating ?
58.
Which of the following pair of compounds is isoelectronic and isostructural ?
59.
Consider the reaction
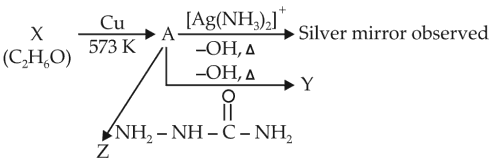
Identify A, X, Y, and Z

Identify A, X, Y, and Z
60.
Which one is the most acidic compound ?
61.
Name the gas that can readily decolourizes acidified KMnO4 solution.
62.
Which one is the correct order of acidity ?
63.
Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.2 × 10–4mol L–1. Solubility product of Ag2C2O4 is
64.
With respect to the conformers of ethane, which of the following statements is true ?
65.
The correct statement regarding electrophile is
66.
Which one is the wrong statement ?
67.
The species, having bond angles of 120° is
68.
The correct increasing order of basic strength for the following compounds is
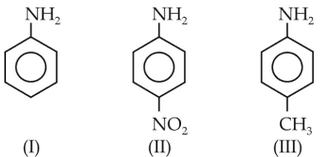

69.
A gas is allowed to expand in a well insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy \(\Delta \text{U}\) of the gas in joules will be
70.
A 20 litre container at of 400 K contains CO2(g) at pressure 0.4 atm and an excess SrO (neglect the volume of solid SrO). The volume of the containers is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of CO2 attains its maximum value, will be
[Given : SrCO3(s) \(\rightleftharpoons\) SrO(s) + CO2 (g).
K = 1.6 atm]
[Given : SrCO3(s) \(\rightleftharpoons\) SrO(s) + CO2 (g).
K = 1.6 atm]
71.
Which of the following statements is not correct ?
72.
Mechanism of a hypothetical reaction X2 + Y2 \(\rightarrow\) 2XY is given below:
(i) X2 \(\rightarrow\) X + X (fast)
(ii) X + Y2 \(\rightleftharpoons\) XY + Y (slow)
(iii) X + Y \(\rightarrow\) XY (fast)
The overall order of the reaction will be
(i) X2 \(\rightarrow\) X + X (fast)
(ii) X + Y2 \(\rightleftharpoons\) XY + Y (slow)
(iii) X + Y \(\rightarrow\) XY (fast)
The overall order of the reaction will be
73.
In which pair of ions both the species contain S – S bond ?
74.
Mixture of chloroxylenol and terpineol acts as
75.
It is because of inability of ns2 electrons of the valence shell to participate in bonding that
76.
For a given reaction, \(\Delta \text{H}\) = 35.5 kJ mol–1 and \(\Delta \text{S}\) = 83.6 JK–1 mol–1. The reaction is spontaneous at :
[Assume that \(\Delta \text{H}\) and \(\Delta \text{S}\) do not vary with temperature]
[Assume that \(\Delta \text{H}\) and \(\Delta \text{S}\) do not vary with temperature]
77.
If molality of the dilute solution is doubled, the value of molal depression constant (Kf ) will be
78.
Which of the following is dependent on temperature ?
79.
Pick out the correct statement with respect to [Mn(CN)6]3–
80.
Which is the incorrect statement ?
81.
Extraction of gold and silver involves leaching with CN- ion. Silver is later recovered by
82.
The correct order of the stoichiometries of AgCl formed when AgNO3 in excess istreated with the complexes:
CoCl3.6NH3, CoCl3.5NH3, CoCl3 .4NH3 respectively is
CoCl3.6NH3, CoCl3.5NH3, CoCl3 .4NH3 respectively is
83.
Identify A and predict the type of reaction
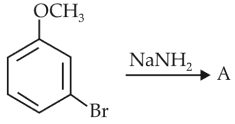

84.
An example of a sigma bonded organometallic compound is
85.
The reason for greater range of oxidation states in actinoids is attributed to
86.
The element Z = 114 has been discovered recently. It will belong to which of the following family group and electronic configuration ?
87.
A first order reaction has a specific reaction rate of 10-2 s-1. How much time will it take for 20 g of the reactant to reduce to 5 g ?
88.
Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field ?
89.
In the electrochemical cell
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)
90.
Which one of the following statements is not correct ?
91.
The final proof for DNA as the genetic material came from the experiments of
92.
In case of poriferans, the spongocoel is lined with flagellated cells called
93.
During DNA replication, Okazaki fragments are used to elongate
94.
Which of the following options best represents the enzyme composition of pancreatic juice ?
95.
Double fertilization is exhibited by
96.
Life cycle of Ectocarpus and Fucus respectively are
97.
Good vision depends on adequate intake of carotene rich food.
Select the best option from the following statements:
(i) Vitamin A derivatives are formed from carotene
(ii) The photopigments are embedded in the membrane discs of the inner segment
(iii) Retinal is a derivative of vitamin A
(iv) Retinal is a light absorbing part of all the visual photopigments
Select the best option from the following statements:
(i) Vitamin A derivatives are formed from carotene
(ii) The photopigments are embedded in the membrane discs of the inner segment
(iii) Retinal is a derivative of vitamin A
(iv) Retinal is a light absorbing part of all the visual photopigments
98.
Attractants and rewards are required for
99.
The vascular cambium normally gives rise to
100.
A baby boy aged two years is admitted to play school and passes through a dental check - up. The dentist observed that the boy had twenty teeth. Which teeth were absent ?
101.
DNA fragments are
102.
Plants which produce characteristic pneu-matophores and show vivipary belongs to
103.
Which of the following options gives the correct sequence of events during mitosis ?
104.
A disease caused by an autosomal primary non- disjunction is
105.
Spliceosomes are not found in cells of
106.
The pivot joint between atlas and axis is a type of
107.
The association of histone H1 with a nucleosome indicates
108.
Which of the following is made up of dead cells ?
109.
Select the correct route for the passage of sperms in male frogs
110.
Adult human RBC's are enucleate. Which of the following statement(s) is/are most appropriate explanation for this feature ?
(i) They do not need to reproduce
(ii) They are somatic cells
(iii) They do not metabolise
(iv) All their internal space is available for oxygen
(i) They do not need to reproduce
(ii) They are somatic cells
(iii) They do not metabolise
(iv) All their internal space is available for oxygen
111.
Homozygous pure lines in cattle can be obtained by
112.
A temporary endocrine gland in the human body is
113.
Viroids differ from viruses in having
114.
A decrease in blood pressure/volume will not cause the release of
115.
An example of colonial alga is
116.
The morphological nature of the edible part of coconut is
117.
Which of the following is correctly matched for the product produced by them ?
118.
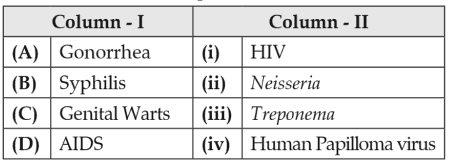
Match the following sexually transmitted diseases (Column - I) with their causative agent (Column - II) and select the correct option.

119.
Among the following characters, which one was not considered by Mendel in his experiments on pea ?
120.
Identify the wrong statement in context of heartwood
121.
Mycorrhizae are the example of
122.
Which of the following RNA's is the most abundant in animal cell ?
123.
The process of separation and purification of expressed protein before marketing is called
124.
Which among the following are the smallest living cells, known without a definite cell wall, pathogenic to plants as well as animals and can survive without oxygen ?
125.
Which of the following components provides sticky character to the bacterial cell ?
126.
With reference to factors affecting the rate of photosynthesis, which of the following statements is not correct ?
127.
Which one of the following statements is correct, with reference to enzymes ?
128.
If there are 999 bases in an RNA that codes for a protein with 333 amino acids, and the base at position 901 is deleted such that the length of the RNA becomes 998 bases, how many codons will be altered ?
129.
Asymptote in a logistic growth curve is obtained when
130.
Select the mismatch from the following
131.
Anaphase promoting complex (APC) is a protein degradation machinery necessary for proper mitosis of animal cells. If APC is defective in a human cell, which of the following is expected to occur ?
132.
Which ecosystem has the maximum biomass ?
133.
Zygotic meiosis is characteristic of
134.
Hypersecretion of Growth Hormone in adults does not cause further increase in height, because
135.
Frog's heart when taken out of the body continues to beat for sometime.
Select the best option from the following statements :
(i) Frog is a poikilotherm
(ii) Frog does not have any coronary circulation
(iii) Heart is "myogenic" in nature
(iv) Heart is autoexcitable
Select the best option from the following statements :
(i) Frog is a poikilotherm
(ii) Frog does not have any coronary circulation
(iii) Heart is "myogenic" in nature
(iv) Heart is autoexcitable
136.
Transplantation of tissues/organs fails often due to non-acceptance by the patient's body. Which type of immune-response is responsible for such rejections ?
137.
Thalassemia and sickle cell anaemia are caused due to a problem in globin molecule synthesis. Select the correct statement.
138.
An important characteristic that Hemichordates share with Chordates is
139.
Which of the following cell organelles is responsible for extracting energy from carbohydrates to form ATP ?
140.
Lungs are made up of air-filled sacs, the alveoli. They do not collapse even after forceful expiration, because of
141.
Which of the following are not polymeric ?
142.
Flowers which have single ovule in the ovary and are packed into inflorescence are usually pollinated by
143.
Presence of plants arranged into well defined vertical layers depending on their height can be seen best in
144.
Phosphoenol pyruvate (PEP) is the primary CO2 acceptor in
145.
Which one from the given below is the period for Mendel's hybridization experiments ?
146.
Select the mismatch
147.
In case of a couple, where the male is having a very low sperm count, which technique will be suitable for fertilisation ?
148.
Which among these is the correct combination of aquatic mammals ?
149.
Functional megaspore in an angiosperm develops into
150.
Root hairs develop from the region of
151.
A dioecious flowering plant prevents both
152.
The hepatic portal vein drains blood to liver from
153.
What is the criterion for DNA fragments movement on agarose gel during gel electrophoresis ?
154.
Which of the following represents order of ' Horse ' ?
155.
Which statement is wrong for Kreb's cycle ?
156.
Artificial selection to obtain cows yielding higher milk output represents
157.
The region of Biosphere Reserve which is legally protected and where no human activity is allowed is known as
158.
Receptor sites for neurotransmitters are present on
159.
The water potential of pure water is
160.
Capacitation occurs in
161.
The function of copper ions in copper releasing IUD's is
162.
A gene whose expression helps to identify transformed cell is known as
163.
Which one of the following statements is not valid for aerosols ?
164.
Which of the following statements is correct ?
165.
Which of the following in sewage treatment removes suspended solids ?
166.
GnRH, a hypothalamic hormone, needed in reproduction, acts on
167.
Which of the following facilitates opening of stomatal aperture ?
168.
The genotypes of a Husband and Wife are IAIB and IAi. Among the blood types of their children, how many different genotypes and phenotypes are possible ?
169.
Alexander Von Humboldt described for the first time
170.
DNA replication in bacteria occurs
171.
MALT constitutes about ________________ percent of the lymphoid tissue in human body.
172.
In Bougainvillea thorns are the modifications of
173.
Fruit and leaf drop at early stages can be prevented by the application of
174.
Which of the following are found in extreme saline conditions ?
175.
Coconut fruit is a
176.
The DNA fragments separated on an agarose gel can be visualised after staining with
177.
Out of 'X' pairs of ribs in humans only 'Y' pairs are true ribs. Select the option that correctly represents values of X and Y and provides their explanation
178.
Myelin sheath is produced by
179.
Which cells of 'Crypts of Lieberkuhn' secrete antibacterial lysozyme ?
180.
Which one of the following is related to Ex-situ conservation of threatened animals and plants ?