Home >> NEET - 2020
Welcome and Shine in NEET with Shine NEET SmartPrep!.
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Your Full Test Performance Summary
Questions Available: 180
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
1.
The average thermal energy for a monoatomic gas is (where,\(k_ B\) is Boltzmann constant and T is absolute temperature.)
2.
A long solenoid of 50 cm length having 100 turns carries a current of 2.5 A. The magnetic field at the centre of the solenoid is: (\(\mu_0\, =\, 4\, \pi \times 10^{−7}\,T\, m\,A^{−1}\))
3.
A body weighs \(72\,N\) on the surface of the earth. What is the gravitational force on it, at a height equal to half the radius of the earth?
4.
Dimensions of stress are :
5.
Taking into account of the significant figures, what is the value of 9.99 m − 0.0099 ?
6.
A screw gauge has least count of 0.01 mm and there are 50 divisions in its circular scale. The pitch of the screw gauge is :
7.
Two cylinders A and B of equal capacity are connected to each other via a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stop cock is suddenly opened. The process is
8.
A ball is thrown vertically downward with a velocity of 20m ∕ s from the top of a tower. It hits the ground after some time with a velocity of80m ∕ sThe height of the tower is : (g = 10m ∕ s2)
9.
The mean free path l for a gas,with molecular diameter d and number density n can be expressed as
10.
A cylinder contains hydrogen gas at pressure of 249 kPa and temperature 27°C. Its density is (\(R = 8.3\, J mol^{ −1} K^{−1}\) )
11.
An electron is accelerated from rest through a potential difference of V
volt. If the de Broglie wavelength of the electron is \(1.227 × 10^2nm\) the
potential difference is:
12.
Light of frequency 1.5 times the threshold frequency is incident on a
photosensitive material. What will be the photoelectric current if the
frequency is halved and intensity is doubled?
13.
A capillary tube of radius r is immersed in water and water rises in it to a height h. The mass of the water in the capillary is 5g. Another capillary tube of radius 2r is immersed in water. The mass of water that will rise in this tube is :
14.
The increase in the width of the depletion region in a p-n junction diodeis due to:
15.
For transistor action, which of the following statements is correct?
16.
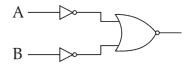
For the logic circuit shown, the truth table is:

17.
A series LCR circuit is connected to an ac voltage source. When L is removed from the circuit, the phase difference between current and voltage is \(\pi /3\). If instead C is removed from the circuit, the phase difference is again \(\pi /3\) between current and voltage. The power factor of the circuit is:
18.
A wire of length L, area of cross-section A is hanging from a fixed support. The length of the wire changes to L1 when mass M is suspended from its free end. The expression for Young’s modulus is
19.
The phase difference between displacement and acceleration of a particle in a simple harmonic motion is:
20.
For which one of the following, Bohr model is not valid?
21.
In a guitar, two strings \(A\) and \(B\) made of same material are slightly out of tune and produce beats of frequency \(6\,Hz\). When tension in \(B\) is slightly decreased, the beat frequency increases to \(7\,Hz\). If the frequency of \(A\) is \(530\,Hz\), the original frequency of \(B\) will be
22.
The energy equivalent of 0.5 g of a substance is
23.
When a uraniumisotope \(\displaystyle {}^{235}_92U\) is bombarded with a neutron, itgenerates \(\displaystyle {}^{89}_{36}Kr\), three neutrons and
24.
A spherical conductor of radius 10 cm has a charge of \(3.2 × 10^{−7} C\) distributed uniformly. What is the magnitude of electric field at a point 15cm from the centre of the sphere? \(\displaystyle \frac{1}{ 4\, \pi\, \epsilon_0} = 9 × 10^9 N m^2/C^2\))
25.
In a certain region of space with volume 0.2 m3, the electric potential is found to be 5 V through out. The magnitude of electric field in this region is:
26.
A short electric dipole has a dipole moment of \(16 \times 10^{−9}\) Cm. The electric potential due to the dipole at a point at a distance of 0.6 m from the centre of the dipole, situated on a line making an angle of \(60^\circ\) with the dipole axis is : \(\displaystyle \left(\frac{1}{4πϵ_0} = 9 × 10^9 Nm^2/C^2\right)\)
27.
The capacitance of a parallel plate capacitor with air as medium is 6µF . With the introduction of a dielectric medium, the capacitance becomes 30µF. The permittivity of the medium is:
(\(E_0 = 8.85 × 10^{−12}\,C^2N^{−1}m^{−2}\))
(\(E_0 = 8.85 × 10^{−12}\,C^2N^{−1}m^{−2}\))
28.
Assume that light of wavelength 600 nm is coming from a star. The limit of resolution of telescope whose objective has a diameter of 2 m is
29.
A ray is incident at an angle of incidence i on one surface of a small angle prism (with angle of prism A) and emerges normally from the opposite surface. If the refractive index of the material of the prism is \(\mu\), then the angle of incidence is nearly equal to
30.
Two particles of mass 5 kg and 10 kg respectively are attached to the two ends of a rigid rod of length 1 m with negligible mass.The centre of mass of the system from the 5 kg particle is nearly at a distance of
31.
Two bodies of mass 4 kg and 6 kg are tied to the ends of a massless string. The string passes over a pulley which is frictionless (see figure). The acceleration of the system in terms of acceleration due to gravity (g) is
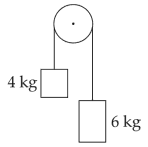

32.
In Young's double slit experiment, if the separation between coherent sources is halved and the distance of the screen from the coherent sources is doubled, then the fringe width becomes
33.
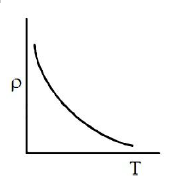
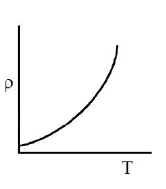
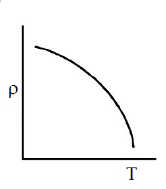
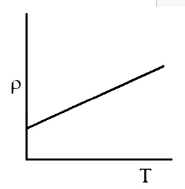
Which of the following graph represents the variation of resistivity (\(\rho\)) with temperature (T) for copper?
34.
Light with an average flux of \(20\, \text{W}/cm^2\) falls on a non-reflecting surface at normal incidence having surface area \(20\,cm^2\). The energy recieved by the surface during time span of 1 minute is
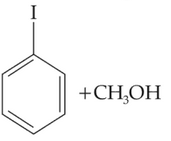
35.
The solids which have the negative temperature coefficient of resistanceare:
36.
An iron rod of susceptibility \(599\) is subjected to a magnetising field of \(1200\,A\,m^{−1}\). The permeability of the material of the rod is :\(\mu_0\, =\, 4\, \pi \times 10^{−7}\,T\, m\,A^{−1}\)
37.
The quantities of heat required to raise the temperarture of two solid copper spheres of radii \(r_1\) and \(r_2\) (\(r_1\,=\,1.5\,r_2\)) through 1 K are in the ratio
38.
The color code of a resistance is given below Yellow Violet Brown GoldThe values of resistance and tolerance, respectively, are 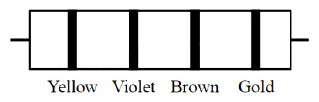

39.
Find the torque about the origin when a force of \(3\, \hat{j}\, \text{N}\) acts on a particle whose position vector is \(2\, \hat{k}\, m\).
40.
A charged particle having drift velocity of \(7.5 \times 10^{−4}\,ms^{−1}\) in an electric field of \(3 \times 10^{−10}\, V\, m^{−1}\), has a mobility in \(m^2\,V^{−1}\,s^{−1}\) of
41.
The Brewsters angle \(i_b\) for an interface should be
42.
A resistance wire connected in the left gap of a metre bridge balances a \(10\, \Omega\) resistance in the right gap at a point which divides the bridge wire in the ratio \(3 : 2\). If the length of the resistance wire is \(1.5\, m\), then the length of \(1\, \Omega\) of the resistance wire is:
43.
The energy required to break one bond in DNA is \(10^{-20}\, \text{J}\). This value in eV is nearly
44.
The ratio of contributions made by the electric field and magnetic field components to the intensity of an electromagnetic wave is : ( c = speed of electromagnetic waves)
45.
A 40μF capacitor is connected to a 200 V, 50 Hz AC supply. The rms value of the current in the circuit is, nearly
46.
What is the change in oxidation number of carbon in the following reaction ?
\(\text{CH}_4\text{(g)} \,+\, \text{4Cl}_2\text{(g)} \, \rightarrow \, \text{CCl}_4\text{(i)}\, +\, \text{4HCl(g)}\)
\(\text{CH}_4\text{(g)} \,+\, \text{4Cl}_2\text{(g)} \, \rightarrow \, \text{CCl}_4\text{(i)}\, +\, \text{4HCl(g)}\)
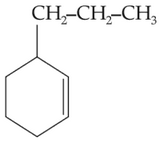
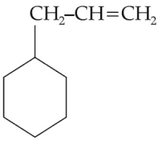
47.
Which of the following alkane cannot be made in good yield by Wurtz reaction?
48.
An element has a body centered cubic (bcc)
structure with a cell edge of 288 pm. The atomic
radius is:
49.
The number of protons, neutrons and electrons in
\(^{175} _{71}Lu\), respectively are:
50.
An alkene on ozonolysis gives methanal as one of
the product. Its structure is :
51.
On electrolysis of dilute suiphuric acid using Platinum
(Pt) electrode, the product obtained at anode will be:
52.
An increase in the concentration of the reactants
of a reaction leads to change in:
53.
Reaction between benzaldehyde and acetophenone
in presence of dilute NaOH is known as:
54.
Which of the following is a natural polymer?
55.
A mixture of N2 and Ar gases in a cylinder contains 7g of N2 and 8g of Ar. If the total pressure of the mixture of the gases in cylinder is 27 bar, the partial pressure of N2 is:
[Use atomic masses (in gmol-1): N = 14, Ar = 40]
[Use atomic masses (in gmol-1): N = 14, Ar = 40]
56.
Match the following and identify the correct option.
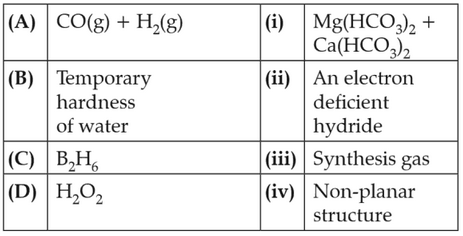

57.
For the reaction, 2Cl(g)→ C2(g), the correct option is:
58.
Urea reacts with water to form A which will
decompose to form B. B when passed through Cu2+ (aq), deep blue colour solution C is formed. What is the formula of C from the following ?
59.
Reaction between acetone and methyl magnesium chloride followed by hydrolysis will give:
60.
The following metal ion activates many enzymes,
participates in the oxidation of glucose to produce
ATP and with Na, is responsible for the transmission
of nerve signals.
61.
Which of the following set of molecules will have
zero dipole moment?
62.
Identify a molecule which does not exist.
63.
ldentify the incorrect match.

64.
The rate constant for a first order reaction is
4.606 x 10-3s-1, The time required to reduce 2.0g of the reactant to 0.2 g is:
65.
ldentify the correct statements from the folowing:
66.
Measuring Zeta potential is useful in determining
which property of colloidal solution ?
67.
Which of the following oxoacid of sulphur has -O-O-linkage?
68.
Elimination reaction of 2-Bromo-pentane to form
pent-2-ene is:
(a) \(\beta\) - Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
Choose the correct option from the following:
(a) \(\beta\) - Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
Choose the correct option from the following:
69.
ldentify the correct statements from the following:
(a) CO2(g) is used as refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains twelve six carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert alcohols into gasoline.
(d) CO is colorless and odourless gas.
(a) CO2(g) is used as refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains twelve six carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert alcohols into gasoline.
(d) CO is colorless and odourless gas.
70.
Paper chromatography is an example of:
71.
Match the following :
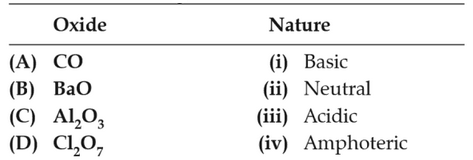

72.
Which one of the followings has maximum number
of atoms ?
73.
Which of the following is a basic amino acid ?
74.
The calculated spin only magnetic moment of Cr2+ ion is:
75.
Sucrose on hydrolysis gives:
76.
The mixture which shows positive deviation from
Raoult's law is:
77.
A tertiary butyl carbocation is more stable than a secondary butyl carbocation because of which of
the following ?
78.
Find out the solubility of Ni(OH)2 in 0.1 M NaOH. Given that the ionic product of Ni(OH)2 is 2 x 10-15
79.
Which of the following is a cationic detergent ?
80.
The freezing point depression constant (Kf) of benzene is 5.12 K kg mol-1. The freezing point depression for the solution of molality 0.078 m containing a non-electrolyte solute in benzene is
(rounded off up to two decimal places):
(rounded off up to two decimal places):
81.
ldentify the incorrect statement.
82.
Which of the following is not correct about carbon monoxide ?
83.
Hydrolysis of sucrose is given by the following reaction.
Sucrose + H2O \(\rightleftharpoons \) Glucose + Fructose
If the equilibrium constant (Kc) is 2 x 1013 at 800 K, the value of \(\Delta \text{rG}^ \ominus \) at the same temperature will be
Sucrose + H2O \(\rightleftharpoons \) Glucose + Fructose
If the equilibrium constant (Kc) is 2 x 1013 at 800 K, the value of \(\Delta \text{rG}^ \ominus \) at the same temperature will be
84.
Which of the following is the correct order of
increasing field strength of ligands to form
coordination compounds ?
85.
ldentify compound X in the following sequence of
reacions:
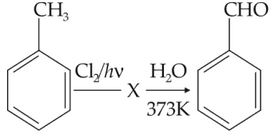

86.
The correct option for free expansion of an ideal
gas under adiabatic condition is:
87.
The number of Faradays(F) required to produce
20 g of calcium from molten CaCl2 (Atomic mass of Ca = 40 g mol-1) is:
88.
HCl was passed through a solution of CaCI2, MgCi2 and NaCI. Which of the following compound(s) crystallise(s)?
89.
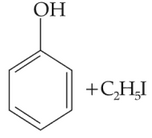
Anisole on cleavage with Hl gives:
90.
Which of the following amine will give the
carbylamine test ?
91.
Which of the following refer to correct example(s)
of organisms which have evolved due to changes in
environment brought about by anthropogenic action
(a) Darwin's Finches of Galapagos islands.
(b) Herbicide resistant weeds.
(c) Drug resistant eukaryotes.
(d) Man-created breeds of domesticated animals like dogs.
(a) Darwin's Finches of Galapagos islands.
(b) Herbicide resistant weeds.
(c) Drug resistant eukaryotes.
(d) Man-created breeds of domesticated animals like dogs.
92.
Which of the following statements are true for the
phylum-Chordata?
(a) In Urochordata notochord extends from head to tail and it is present throughout their life.
(b) In Vertebrata notochord is present during the embryonic period only.
(c) Central nervous system is dorsal and hollow.
(d) Chordata is divided into 3 subphyla - Hemichordata, Tunicata and Cephalochordata.
(a) In Urochordata notochord extends from head to tail and it is present throughout their life.
(b) In Vertebrata notochord is present during the embryonic period only.
(c) Central nervous system is dorsal and hollow.
(d) Chordata is divided into 3 subphyla - Hemichordata, Tunicata and Cephalochordata.
93.
Presence of which of the following conditions in
urine are indicative of Diabetes Mellitus?
94.
Select the option including all sexually transmitted
diseases.
95.
Match the following columns and select the correctoption.


96.
If the distance between two consecutive base pairs
is 0.34 nm and the total number of base pairs of a
DNA double helix in a typical mammalian cell is
6.6 x 109 bp, then the length of the DNA is
approximately:
97.
The enzyme enterokinase helps in conversion of:
98.
Identify the correct statement with reference to
human digestive system.
99.
ldentify the wrong statement with regard to
Restriction Enzymes.
100.
Match the following:
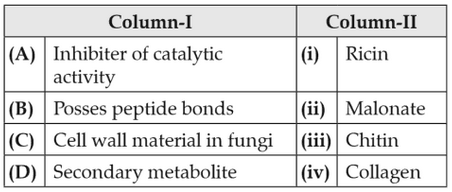
Choose the correct option from the following:

Choose the correct option from the following:
101.
Dissolution of the synaptonemal complex occurs
during:
102.
Name the enzyme that facilitates opening of DNA
helix during transcription.
103.
Match the following columns and select the correctoption.
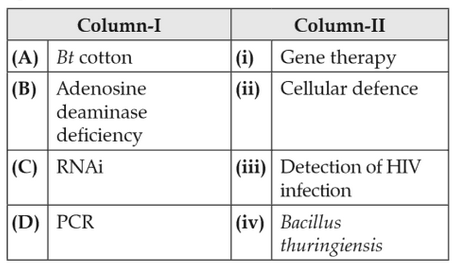

104.
ldentify the wrong statement with reference to
transport of oxygen.
105.
Which of the following is not an inhibitory substance
governing seed dormancy ?
106.
Match the following diseases with the causativeorganism and select the correct option.
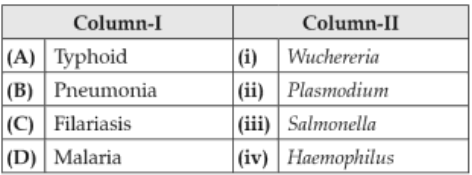

107.
Select the correct events that occur during inspiration.
(a) Contraction of diaphragm
(b) Contraction of external inter-costal muscles
(c) Pulmonary volume decreases
(d) Intra pulmonary pressure increases
(a) Contraction of diaphragm
(b) Contraction of external inter-costal muscles
(c) Pulmonary volume decreases
(d) Intra pulmonary pressure increases
108.
The oxygenation activity of RuBisCo enzyme in
photorespiration leads to the formation of:
109.
In light reaction, plastoquinone facilitates the
transfer of electrons from:
110.
In gel electrophoresis, separated DNA fragments can be visualized with the help of:
111.
The QRS complex in a standard ECG represents:
112.
The plant parts which consist of two generations-
one within the other:
(a) Pollen grains inside the anther
(b) Germinated pollen grain with two male gametes
(c) Seed inside the fruit
(d) Embryo sac inside the ovule
(a) Pollen grains inside the anther
(b) Germinated pollen grain with two male gametes
(c) Seed inside the fruit
(d) Embryo sac inside the ovule
113.
The infectious stage of Plasmodium that enters the
human body is:
114.
ldentify the incorrect statement.
115.
Flippers of Penguins and Dolphins are examples
of:
116.
ldentify the wrong statement with reference to the
gene 'l' that controls ABO blood groups.
117.
The first phase of translation is:
118.
Ray florets have:
119.
The process of growth is maximum during:
120.
The roots that originate from the base of the stem
are
121.
In water hyacinth and water lily, pollination takes
place by:
122.
Which of the following is put into Anaerobic sludge
digester for further sewage treatment ?
123.
Bilaterally symmetrical and acoelomate animals
are exemplified by:
124.
ldentify the basic amino acid from the following.
125.
In which of the following techniques, the embryos
are transferred to assist those females who cannot
conceive?
126.
Which of the following statements about inclusion
bodies is incorrect?
127.
Experimental verification of the chromosomal theory
of inheritance was done by:
128.
Which of the following statements is not correct?
129.
Which is the important site of formation of
glycoproteins and glycolipids in eukaryotic cells ?
130.
Embryological support for evolution was disapproved
by:
131.
The sequence that controls the copy number of
the linked DNA in the vector, is termed:
132.
Which of the following is correct about viroids ?
133.
Montreal protocol was signed in 1987 for control of:
134.
The number of substrate level phosphorylations in
one turn of citric acid cycle is:
135.
Which of the following hormone levels will cause
release of ovum (ovulation) from the graffian follicle?
136.
Select the correct match.
137.
Cuboidal epithelium with brush border of microvilli
is found in:
138.
Snow-blindness in Antarctic region is due to:
139.
Which of the following pairs is of unicellular algae?
140.
The transverse section of a plant shows following
anatomical features:
(a) Large number of scattered vascular bundles surrounded by bundle sheath.
(b) Large conspicuous parenchymatous ground tissue.
(c) Vascular bundles conjoint and closed.
(d) Phloem parenchyma absent.
ldentify the category of plant and its part:
(a) Large number of scattered vascular bundles surrounded by bundle sheath.
(b) Large conspicuous parenchymatous ground tissue.
(c) Vascular bundles conjoint and closed.
(d) Phloem parenchyma absent.
ldentify the category of plant and its part:
141.
How many true breeding pea plant varieties did
Mendel select as pairs, which were similar except
in one character with contrasting traits ?
142.
Floridean starch has structure similar to:
143.
ldentify the correct statement with regard to G1 phase (Gap 1) of interphase.
144.
By which method was a new breed 'Hisardale of
sheep formed by using Bikaneri ewes and Marino
rams ?
145.
Identify the wrong statement with reference to
immunity.
146.
The specific palindromic sequence which is
recognized by EcoRI is:
147.
If the head of cockroach is removed, it may live for
few days because:
148.
Match the trophic levels with their correct speciesexamples in grassland ecosystem.

Select the correct option:

Select the correct option:
149.
Name the plant growth regulator which upon
spraying on sugarcane crop, increases the length
of stem, thus increasing the yield of sugarcane crop.
150.
Goblet cells of alimentary canal are modified from
151.
Match the following columns and select the correctoption.
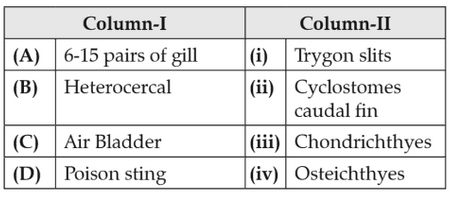

152.
Which of the following statements is correct ?
153.
Which of the following regions of the globe exhibits
highest species diversity ?
154.
Match the following columns and select the correctoption.


155.
The product(s) of reaction catalyzed by nitrogenase
in root nodules of leguminous plants is/are:
156.
Match the following concerning essential elementsand their functions in plants:


157.
Which of the following would help in prevention of
diuresis?
158.
Meiotic division of the secondary oocyte is completed:
159.
Match the following columns and select the correctoption.


160.
Match the following columns and select the correctoption.
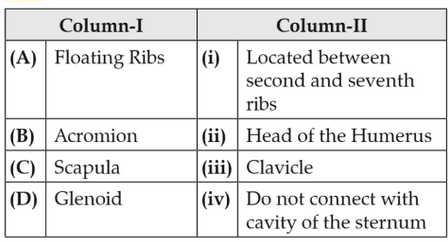

161.
Secondary metabolites such as nicotine, strychnine
and caffeine are produced by plants for their:
162.
From his experiments, S.L. Miller produced amino
acids by mixing the following in a closed flask
163.
Match the organism with its use in biotechnology.

Select the correct option from the following:

Select the correct option from the following:
164.
Bt cotton variety that was developed by the
halten introduction of toxin gene of Bacillus thuringiensis
(Bt) is resistant to
165.
Choose the correct pair from the following:
Break the DNA into
166.
The body of the ovule is fused within the funicle at
167.
Strobili or cones are found in
168.
Match the following columns and select the correctoption.


169.
ldentify the substances having glycosidic bond and
peptide bond, respectively in their structure
170.
In relation to Gross primary productivity and Net
primary productivity of an ecosystem, which one of
the following statements is correct?
171.
Match the following columns and select the correctoption.


172.
Which of the following is not an attribute of a
population ?
173.
Match the following columns and select the correctoption.
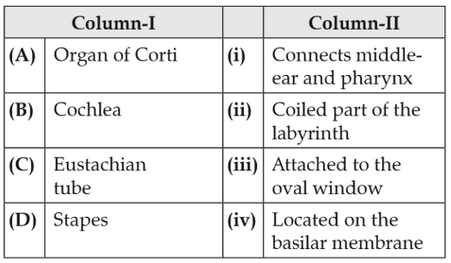

174.
Which one of the following is the most abundant
protein in the animals ?
175.
85.Match the following with respect to meiosis
(a) Zygotene | (i) Terminalization
(b) Pachytene | (ii) Chiasmata(c) Diplotene | (iii) Crossing over
(d) Diakinesis | (iv) Synapsis
Select the correct option from the following:
(a) Zygotene | (i) Terminalization
(b) Pachytene | (ii) Chiasmata(c) Diplotene | (iii) Crossing over
(d) Diakinesis | (iv) Synapsis
Select the correct option from the following:
176.
According to Robert May, the global species diversity is about:
177.
The ovary is half inferior in:
178.
Select the correct statement.
179.
The process responsible for facilitating loss of water
in liquid form from the tip of grass blades at night
and in early morning is
180.
Some dividing cells exit the cell cycle and enter
vegetative inactive stage. This is called quiescent
stage (G). This process occurs at the end of: