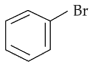
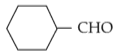
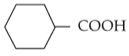
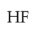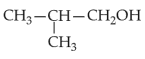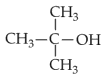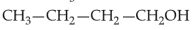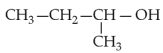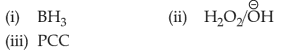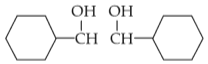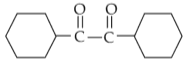Home >> NEET - 2024
Welcome and Shine in NEET with Shine NEET SmartPrep!.
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Your Full Test Performance Summary
Questions Available: 200
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
1.
The moment of inertia of a thin rod about an axis passing through its mid point and perpendicular to the rod is 2400 gcm2. The length of the 400 g rod is nearly:
2.
A particle moving with uniform speed in a circular path maintains:
3.
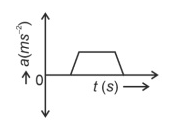
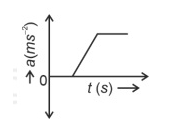
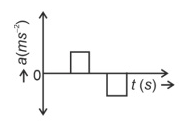
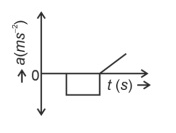
The velocity (v)− time (t) plot of the motion of a body is shown below:
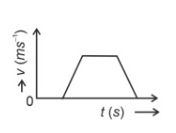
The acceleration (a)− time (t) graph that best suits this motion is :

The acceleration (a)− time (t) graph that best suits this motion is :
4.
The maximum elongation of a steel wire of 1m length if the elastic limit of steel and its Young's modulus, respectively, are 8 × 108 Nm−2 and 2 ×1011 Nm−2, is:
5.
A metallic bar of Young's modulus, \(0.5 × 10^{11}Nm^{−2}\) and coefficient of linear thermal expansion \(10^{−5^\circ}C^{−1}\), length 1m and area of cross section \(10^{−3}m^2\) is heated from \(0^\circ C\) to \(100^\circ C\) with out expansion or bending. The compressive force developed in it is:
6.
A 10μF capacitor is connected to a 210V, 50Hz source as shown in figure. The peak current in the circuit is nearly (π = 3.14) :
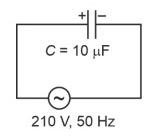

7.
In a vernier callipers, (N + 1) divisions of vernier scale coincide with N divisions of main scale. If 1 MSD represents 0.1mm, the vernier constant (in cm) is:
8.
The quantities which have the same dimensions as those of solid angle are:
9.
A force defined by F = αt2 + βt acts on a particle at a given time t. The factor which is dimensionless, if α and β are constants, is:
10.
Two bodies A and B of same mass undergo completely inelastic one dimensional collision. The body A moves with velocity v1 while body B is at rest before collision. The velocity of the system after collision is v2. The ratio v1 : v2 is
11.
A bob is whirled in a horizontal plane by means of a string with an initial speed of ω rpm.The tension in the string is T. If speed becomes 2ω while keeping the same radius, the tension in the string becomes:
12.
A wheel of a bullock cart is rolling on a level road as shown in the figure below. If its linear speed is ν in the direction shown, which one of the following options is correct (P and Q are any highest and lowest points on the wheel, respectively)?
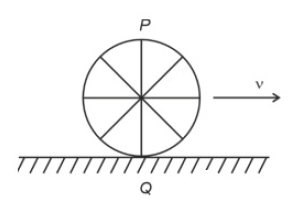

13.
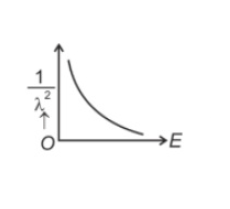
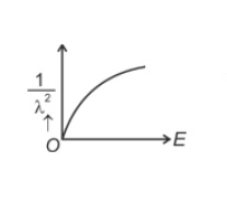
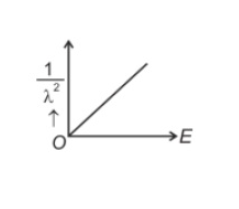
The graph which shows the variation of \((1/λ^2)\) and its kineticenergy, E is (where λ is de Broglie wavelength of a free particle):
14.
If c is the velocity of light in free space, the correct statements about photon among the following are:
A. The energy of a photon is E = hv.
B. The velocity of a photon is c.
C. The momentum of a photon, p = hv/c.
D. In a photon-electron collision, both total energy and total momentum are conserved.
E. Photon possesses positive charge.
Choose the correct answer from the options given below:
A. The energy of a photon is E = hv.
B. The velocity of a photon is c.
C. The momentum of a photon, p = hv/c.
D. In a photon-electron collision, both total energy and total momentum are conserved.
E. Photon possesses positive charge.
Choose the correct answer from the options given below:
15.
A thin flat circular disc of radius 4.5 cm is placed gently over the surface of water. If surface tension of water is 0.07Nm−1, then the excess force required to take it away from the surface is
16.
A logic circuit provides the output Y as per the following truth table :
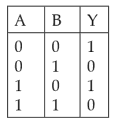
The expression for the output Y is

The expression for the output Y is
17.
Consider the following statements A and B and identify the correct answer:
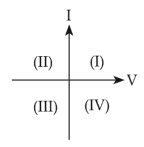
A. For a solar-cell, the I-V characteristics lies in the IV quadrant of the given graph.
B. In a reverse biased pn junction diode, the current measured (in µA), is due to majority charge carriers.

A. For a solar-cell, the I-V characteristics lies in the IV quadrant of the given graph.
B. In a reverse biased pn junction diode, the current measured (in µA), is due to majority charge carriers.
18.
The output (Y) of the given logic gate is similar to the output of an/a
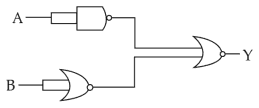

19.
In an ideal transformer, the turns ratio is \(\frac{N_P}{N_S} = \frac{1}{2}\). The ratio \(V_S : V_P\) is equal to (the symbols carry their usual meaning) :
20.
If \(x = 5sin (πt + π/3) m\) represents the motion of a particle executing simple harmonic motion, the amplitude and time period of motion,respectively, are
21.
If the mass of the bob in a simple pendulum is increased to thrice its original mass and its length is made half its original length, then the new time period of oscillation is \(x/2\) times its original time period. Then the value of x is:
22.
A thin spherical shell is charged by some source. The potential difference between the two points C and P (in V ) shown in the figure is: (Take \( \frac{1}{4πϵ0} = 9 × 10^9 \) SI units)
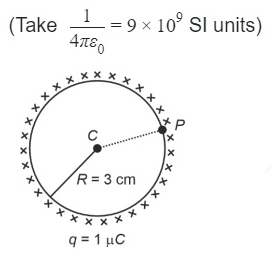

23.
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: The potential (V) at any axial point, at 2 m distance (r) from the centre of the dipole of dipole moment vector \(\vec{P}\) of magnitude, 4 ×10−6Cm , is ± 9 × 103V. (Take \(\frac{1}{4πϵ_0} = 9 × 10^9\) SI units).
Reason R: V = ± \(\frac{2P}{4πϵ_0r^2}\) where r is the distance of any axial point, situated at 2 m from the centre of the dipole. In the light of the above statements, choose the correct answer from the options given below:
Assertion A: The potential (V) at any axial point, at 2 m distance (r) from the centre of the dipole of dipole moment vector \(\vec{P}\) of magnitude, 4 ×10−6Cm , is ± 9 × 103V. (Take \(\frac{1}{4πϵ_0} = 9 × 10^9\) SI units).
Reason R: V = ± \(\frac{2P}{4πϵ_0r^2}\) where r is the distance of any axial point, situated at 2 m from the centre of the dipole. In the light of the above statements, choose the correct answer from the options given below:
24.
In the following circuit, the equivalent capacitance betweenterminal A and terminal B is :
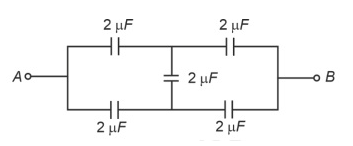

25.
If the plates of a parallel plate capacitor connected to a battery are moved close to each other, then
A. the charge stored in it, increases.
B. the energy stored in it, decreases.
C. its capacitance increases.
D. the ratio of charge to its potential remains the same.
E. the product of charge and voltage increases.
Choose the most appropriate answer from the options given below:
A. the charge stored in it, increases.
B. the energy stored in it, decreases.
C. its capacitance increases.
D. the ratio of charge to its potential remains the same.
E. the product of charge and voltage increases.
Choose the most appropriate answer from the options given below:
26.
A parallel plate capacitor is charged by connecting it to a battery through a resistor. If I is the current in the circuit, then in the gap between the plates:
27.
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is: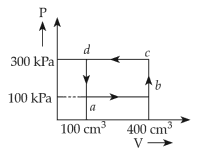

28. 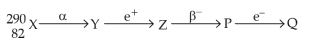
In the nuclear emission stated above, the mass number and atomic number of the product Q respectively, are:
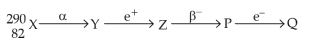
In the nuclear emission stated above, the mass number and atomic number of the product Q respectively, are:
29.
An unpolarised light beam strikes a glass surface at Brewster’s angle. Then
30.
The terminal voltage of the battery, whose emf is 10V and internal resistance 1Ω , when connected through an external resistance of 4Ω asshown in the figure is:
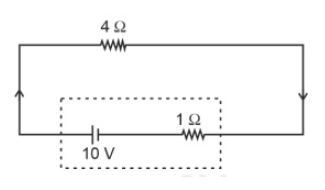

31.
A light ray enters through a right angled prism at point P with the angle of incidence \(30^ \circ\) as shown in figure . It travels through the prism parallel to its base BC and emerges along the face AC . The refractive index of the prism is :
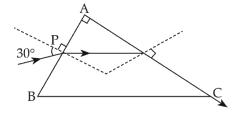

32.
A wire of length 'l' and resistance 100Ω is divided into 10 equal parts.
The first 5 parts are connected in series while the next 5 parts are
connected in parallel. The two combinations are again connected in
series. The resistance of this final combination is:
33.
If the monochromatic source in Young's double slit experiment is replaced by white light, then
34.
A horizontal force 10 N is applied to a block A as shown in figure. The mass of blocks A and B are 2 kg and 3 kg respectively. The blocks slide over a frictionless surface. The force exerted by block A on block B is :
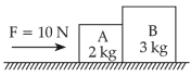

35.
Two heaters A and B have power rating of 1 kW and 2 kW, respectively.
Those two are first connected in series and then in parallel to a fixed
power source. The ratio of power outputs for these two cases is:
36.
Given below are two statements :
Statement I : Atoms are electrically neutral as they contain equal number of positive and negative charges .
Statement II : Atoms of each element are stable and emit their characteristic spectrum .
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Atoms are electrically neutral as they contain equal number of positive and negative charges .
Statement II : Atoms of each element are stable and emit their characteristic spectrum .
In the light of the above statements, choose the most appropriate answer from the options given below :
37.
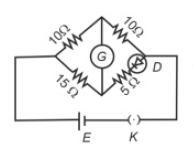
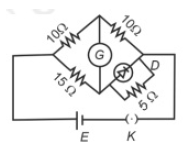
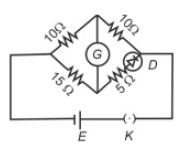
Choose the correct circuit which can achieve the bridge balance.
38.
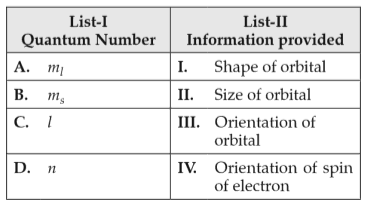
Match List I with List II
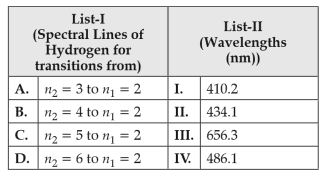
Choose the correct answer from the options given below

Choose the correct answer from the options given below
39.
The following graph represents the T - V curves of an ideal gas ( where T is the temperature and V the volume ) at three pressures P1 , P2 and P3 compared with those of Charles's law represented as dotted lines .
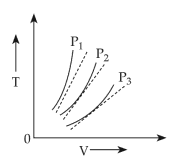

40.
A small telescope has an objective of focal length 140 cm and an eye piece of focal length 5.0 cm . The magnifying power of telescope for viewing a distant object ?
41.
In a uniform magnetic field of 0.049T, a magnetic needle performs 20 complete oscillations in 5 seconds as shown. The moment of inertia of the needle is \(9.8×10^{−6} kgm^2\). If the magnitude of magnetic moment of the needle is \(x×10^{−5} Am^2\), then the value of 'x ' is :
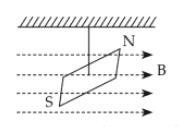

42. 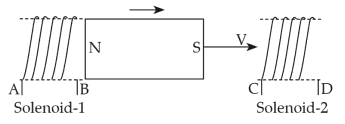
In the above diagram, a strong bar magnet is moving towards solenoid-2 from solenoid-1. The direction of induced current in solenoid-1 and that in solenoid-2, respectively, are through the directions:

In the above diagram, a strong bar magnet is moving towards solenoid-2 from solenoid-1. The direction of induced current in solenoid-1 and that in solenoid-2, respectively, are through the directions:
43.
Match List-I with List-II.
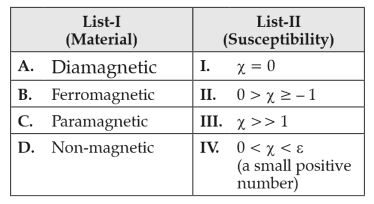
Choose the correct answer from the options given below.

Choose the correct answer from the options given below.
44.
A sheet is placed on a horizontal surface in front of a strong magneticpole. A force is needed to:
A. hold the sheet there if it is magnetic.
B. hold the sheet there if it is non-magnetic.
C. move the sheet away from the pole with uniform velocity if it is conducting.
D. move the sheet away from the pole with uniform velocity if it is both,non-conducting and non-polar.
Choose the correct statement(s) from the options given below:
A. hold the sheet there if it is magnetic.
B. hold the sheet there if it is non-magnetic.
C. move the sheet away from the pole with uniform velocity if it is conducting.
D. move the sheet away from the pole with uniform velocity if it is both,non-conducting and non-polar.
Choose the correct statement(s) from the options given below:
45.
An iron bar of length L has magnetic moment M. It is bent at the middle of its length such that the two arms make an angle \(60^ \circ\) with each other. The magnetic moment of this new magnet is :
46.
The property which is not of an electromagnetic wave travelling in free space is that:
47.
A tightly wound 100 turns coil of radius 10 cm carries a current of 7A. The magnitude of the magnetic field at the centre of the coil is (Take permeability of free space as 4π × 10−7 SI units):
48.
At any instant of time t, the displacement of any particle is given by 2t −1 (SI unit) under the influence of force of 5N. The value of instantaneous power is (in SI unit):
49.
The mass of a planet is \(1/10^{th}\) that of the earth and its diameter is half that of the earth. The acceleration due to gravity on that planet is:
50.
The minimum energy required to launch a satellite of mass \(m\) from the surface of earth of mass \(M\) and radius \(R\) in a circular orbit at an altitude of \(2R\) from the surface of the earth is:
51.
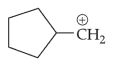
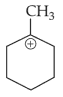
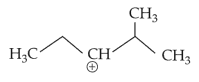
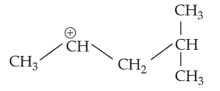
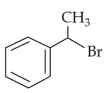
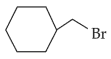
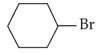
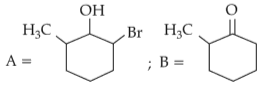
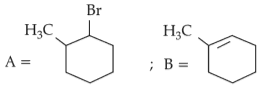
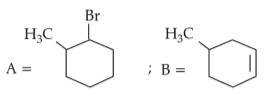
The most stable carbocation among the following is:
52.
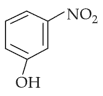
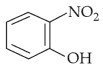
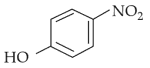
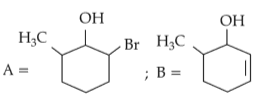
Intramolecular hydrogen bonding is present in
53.
The \(\text{E}^\circ\) value for the \(\text{Mn}^{3+}\,/\,\text{Mn}^{2+}\) couple is more positive than that of \(\text{Cr}^{3+}\,/\,\text{Cr}^{2+}\) or \(\text{Fe}^{3+}\,/\,\text{Fe}^{2+}\) due to change of:
54.
Given below are two statements:
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
55.
Arrange the following elements in increasing order of electronegativity:
N, O, F, C, Si
Choose the correct answer from the options given below
N, O, F, C, Si
Choose the correct answer from the options given below
56.
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
57.
A compound X contains 32 % of A, 20 % of B2 and remaining percentage of C. Then, the empirical formula of X is:
58.
The rate of a reaction quadruples where temperature cahnges from \(27^\circ \text{C}\) to \(57^\circ \text{C}\) calculate the energy of activation.
Given \(\text{R}\,=\,8.314\,\text{J}\,\text{K}^{-1}\,\text{mol}^{-1}\), \(\text{log}4\,=\,0.6021\)
Given \(\text{R}\,=\,8.314\,\text{J}\,\text{K}^{-1}\,\text{mol}^{-1}\), \(\text{log}4\,=\,0.6021\)
59.
The plot of osmotic pressure (\(\pi\)) vs concentration (\(\text{mol L}^{-1}\)) for a solution gives a straight line with slope \(25.73\,\text{L bar mol}^{-1}\). The temperature at which the osmotic pressure measurement is done is
(Use \(\text{R}\,=\, 0.083\,\text{L bar mol}^{-1}\text{K}^{-1}\))
(Use \(\text{R}\,=\, 0.083\,\text{L bar mol}^{-1}\text{K}^{-1}\))
60.
During the preparation of Mohr’s salt solution (Ferrous ammonium sulphate), which of the following acid is added to prevent hydrolysis of \(Fe^{2+}\) ion
61.
Mass in grams of copper deposited by passing 9.6487 A current through a voltmeter containing copper sulphate solution for 100 seconds is:
(Given: Molar mass of \(\text{Cu:}\, 63\,g\,\text{mol}^{-1}\), \(1\text{F}\, =\, 96487 \text{C}\))
(Given: Molar mass of \(\text{Cu:}\, 63\,g\,\text{mol}^{-1}\), \(1\text{F}\, =\, 96487 \text{C}\))
62.
Identify the correct answer
63.
Statement I:
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below
64.
Match List-I with List-II
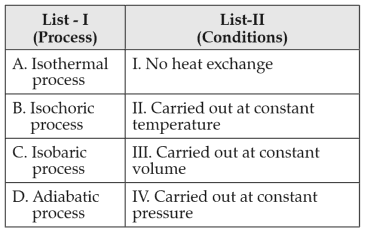
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
65.
Match List-I with List-II
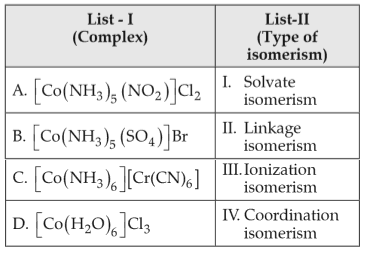
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
66.
On heating, some solid substances change from solid to vapour state without passing through liquid state. The technique used for the purification of such solid substances based on the above principle is konwon as
67.
Match List-I with List-II:
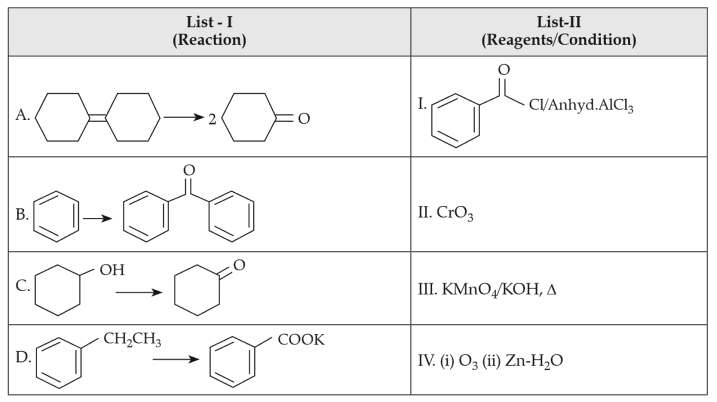

68.
The highest number of helium atoms is in
69.
For the reaction \(2A \rightleftharpoons B + C, \, K_c \, = \, 4 \times 10^{-3}\). At a given time, the composition of reaction mixture is:
\([A]\, =\, [B]\, = \, [C]\, =\, 2 \times 10^{-3}\,M\)
Then, which of the following is correct?
\([A]\, =\, [B]\, = \, [C]\, =\, 2 \times 10^{-3}\,M\)
Then, which of the following is correct?
70.
Fehling’s solution ‘A’ is
71.
Match List-I with List-II
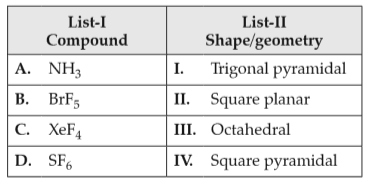
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
72.
Among group 16 elements, which one does NOT show -2 oxidation state
73.
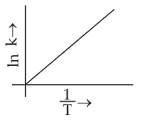
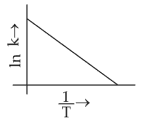
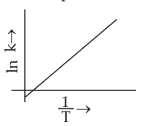
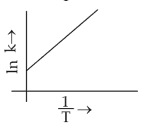
Which plot of \(ln\,k\) vs \(\displaystyle \frac{1}{T}\) is consistent with Arrhenius equation?
74.
Given below are two statements:
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
75.
Which is NOT a redox reaction?
76.
Arrange the following elements in increasing order of first ionization enthalpy:
Li, Be, B, N
Choose the correct answer from the options given below:
Li, Be, B, N
Choose the correct answer from the options given below:
77.
Which of the following alcohols reacts instantaneously with Lucas reagent?
78.
Match List-I with List-II:
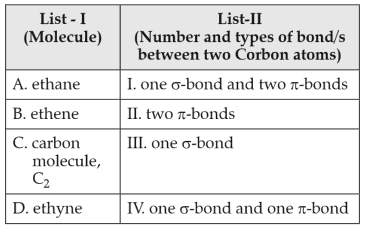
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
79.
Given below are two statements:
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
80.
Given below are two statements:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
81.
Match List-I with List-II:
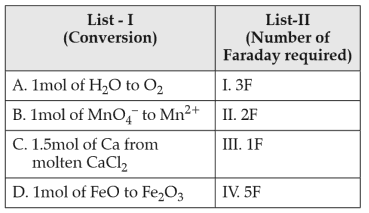
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
82.
In which of the following equilibria, \(\text{K}_\text{P}\) and \(\text{K}_\text{C}\) are NOT equal?
83.
The Henry's law constant \(\left(\text{K}_\text{H}\right)\) values of three gases (A, B, C) in water are \(145\), \(2 \times 10^{-5}\) and \(35\) kbar, respectively. The solubility of these gases in water follow the order:
84.
Identify the correct reagents that would bring about the following transformation.
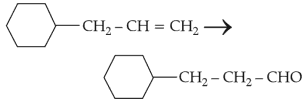

85.
The compound that will undergo \(\text{S}_\text{N}1\) reaction with the fastest rate is:
86.
The energy of an electron in the ground state (n = 1) for \(\text{He}^+\) ion is \(-x\text{J}\), then that for an electron in n = 2 state for \(\text{Be}^{3+}\) ion in J is:
87.
A compound with a molecular formula of \(\text{C}_6\text{H}_{14}\) has two tertiary carbons. Its IUPAC name is:
88.
The reagents with which glucose does not react to give the corresponding tests/products are
A. Tollen's reagent
B. Schiff's reagent
C. HCN
D. NH2OH
E. NaHSO3
Choose the correct options from the given below:
A. Tollen's reagent
B. Schiff's reagent
C. HCN
D. NH2OH
E. NaHSO3
Choose the correct options from the given below:
89.
Spin only' magnetic moment same for which of the following ions?
A. \(\text{Ti}^{3+}\)
B. \(\text{Cr}^{2+}\)
C. \(\text{Mn}^{2+}\)
D. \(\text{Fe}^{2+}\)
E. \(\text{Sc}^{3+}\)
Choose the most appropriate answer from the options given below:
A. \(\text{Ti}^{3+}\)
B. \(\text{Cr}^{2+}\)
C. \(\text{Mn}^{2+}\)
D. \(\text{Fe}^{2+}\)
E. \(\text{Sc}^{3+}\)
Choose the most appropriate answer from the options given below:
90.
Match List-I with List-II
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
91.
1 gram of sodium hydroxide was treated with 25mL of 0.75 M HCl solution, the mass of sodium hydroxide left unreacted is equal to
92.
In which of the following processes entropy form increases ?
93.
Activation energy of any chemical reactions can be calculated if one knows the value of
94.
Major products A and B formed in the following reaction sequence , are
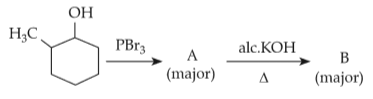

95.
The work done during reversible isothermal expansion of one mole of hydrogen gas at \(25^\circ \text{C}\) from pressure of \(20\) atmosphere to \(10\) atmosphere is:
(Given \(\text{R}\,=\,2.0\,cal K^{-1}mol^{-1}\))
(Given \(\text{R}\,=\,2.0\,cal K^{-1}mol^{-1}\))
96.
Consider the following reaction in a sealed vessel at equilibrium with concentrations of
\(\text{N}_2\,=\,3.0 \times 10^{-3}\text{M}\),
\(\text{O}_2\,=\,4.2 \times 10^{-3}\text{M}\),
\(\text{NO}\,=\,2.8 \times 10^{-3}\text{M}\),
\(2\text{NO}\left(g\right)\,\rightleftharpoons\, \text{N}_2\left(g\right)\,+\, \text{O}_2\left(g\right)\),
If \( 0.1\text{molL}^{-1}\) of \(\text{NO}\left(g\right)\) is taken in a closed vessel, what will be degree of dissociation \(\left(\alpha\right)\) of \(\text{NO}\left(g\right)\) at equilibrium?
\(\text{N}_2\,=\,3.0 \times 10^{-3}\text{M}\),
\(\text{O}_2\,=\,4.2 \times 10^{-3}\text{M}\),
\(\text{NO}\,=\,2.8 \times 10^{-3}\text{M}\),
\(2\text{NO}\left(g\right)\,\rightleftharpoons\, \text{N}_2\left(g\right)\,+\, \text{O}_2\left(g\right)\),
If \( 0.1\text{molL}^{-1}\) of \(\text{NO}\left(g\right)\) is taken in a closed vessel, what will be degree of dissociation \(\left(\alpha\right)\) of \(\text{NO}\left(g\right)\) at equilibrium?
97.
For the given reaction:
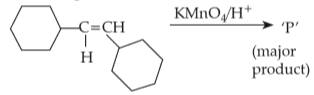
'P' is

'P' is
98.
The pair of lanthanoid ions which are diamagnetic is
99.
Identify the major product C formed in the following reaction sequence:


100.
The products A and B obtained in the following reactions, respectively, are
\(3\text{ROH}\,+\,\text{PCl}_3\,\rightarrow\,3\text{RCl}\,+\,A\)
\(\text{ROH}\,+\,\text{PCl}_5\,\rightarrow\,\text{RCl}\,+\,\text{RCl}\,+\,B\)
\(3\text{ROH}\,+\,\text{PCl}_3\,\rightarrow\,3\text{RCl}\,+\,A\)
\(\text{ROH}\,+\,\text{PCl}_5\,\rightarrow\,\text{RCl}\,+\,\text{RCl}\,+\,B\)
101.
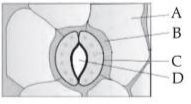
In the given figure, which component has thin outer walls and highly thickened inner walls?

102.
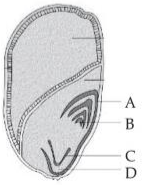
Identify the part of the seed from the given figure which is destined to form root when the seed germinates

103.
These are regarded as major causes of biodiversity loss:
A. Overexploitation
B. Co-extinction
C. Mutation
D. Habitat loss and fragmentation
E. Migration
A. Overexploitation
B. Co-extinction
C. Mutation
D. Habitat loss and fragmentation
E. Migration
104.
Match List I with List II:
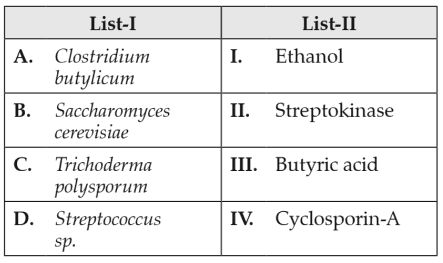
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
105.
Given below are two statements :
Statement I: The cerebral hemispheres are connected by nerve tract known as corpus callosum.
Statement II: The brain stem consists of the medulla oblongata, pons and cerebrum. In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I: The cerebral hemispheres are connected by nerve tract known as corpus callosum.
Statement II: The brain stem consists of the medulla oblongata, pons and cerebrum. In the light of the above statements, choose the most appropriate answer from the options given below :
106.
Identify the correct option (A), (B), ( C), (D) with respect to spermatogenesis.
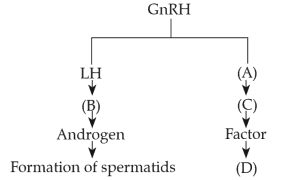

107.
Choose the correct statement given below regarding juxta medullary nephron.
108.
Given below are two statements :
Statement I: Gause’s competitive exclusion principle states that two closely related species competing for different resources cannot exist indefinitely.
Statement II: According to Gause’s principle, during competition, the inferior will be eliminated. This may be true if resources are limiting.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Gause’s competitive exclusion principle states that two closely related species competing for different resources cannot exist indefinitely.
Statement II: According to Gause’s principle, during competition, the inferior will be eliminated. This may be true if resources are limiting.
In the light of the above statements, choose the correct answer from the options given below:
109.
Given below are two statements :
Statement I: Bone marrow is the main lymphoid organ where all blood cells including lymphocytes are produced.
Statement II: Both bone marrow and thymus provide micro environments for the development and maturation of T-lymphocytes.
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement I: Bone marrow is the main lymphoid organ where all blood cells including lymphocytes are produced.
Statement II: Both bone marrow and thymus provide micro environments for the development and maturation of T-lymphocytes.
In the light of the above statements, choose the most appropriate answer from the options given below:
110.
Regarding catalytic cycle of an enzyme action, select the correct sequential steps :
A. Substrate enzyme complex formation.
B. Free enzyme ready to bind with another substrate.
C. Release of products.
D. Chemical bonds of the substrate broken.
E. Substrate binding to active site.
Choose the correct answer from the options given below :
A. Substrate enzyme complex formation.
B. Free enzyme ready to bind with another substrate.
C. Release of products.
D. Chemical bonds of the substrate broken.
E. Substrate binding to active site.
Choose the correct answer from the options given below :
111.
A transcription unit in DNA is defined primarily by the three regions in DNA and these are with respect to upstream and downstream end;
112.
The equation of Verhulst-Pearl logistic growth is -
\(\displaystyle \frac{\text{dN}}{\text{dt}}\,=\,\text{rN}\left[\frac{\text{K}-\text{N}}{\text{K}}\right]\)
From this equation, K indicates:
\(\displaystyle \frac{\text{dN}}{\text{dt}}\,=\,\text{rN}\left[\frac{\text{K}-\text{N}}{\text{K}}\right]\)
From this equation, K indicates:
113.
Inhibition of Succinic dehydrogenase enzyme by malonate is a classical example of:
114.
A pink flowered Snapdragon plant was crossed with a red flowered Snapdragon plant. What type of phenotype/s is/are expected in the progeny?
115.
The type of conservation in which the threatened species are taken out from their natural habitat and placed in special settings where they can be protected and given special care is called:
116.
Which of the following are required for the dark reaction of photosynthesis?
A. Light
B. Chlorophyll
C. CO2
D. ATP
E. NADPH
Choose the correct answer from the options given below:
A. Light
B. Chlorophyll
C. CO2
D. ATP
E. NADPH
Choose the correct answer from the options given below:
117.
Bulliform cells are responsible for-
118.
Identify the type of flowers based on the position of calyx, corolla, and androecium with respect to the ovary from the given figures (a) and (b).
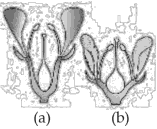

119.
Which one of the following is not a criterion for classification of fungi?
120.
Hind II always cuts DNA molecules at a particular point called recognition sequence and it consists of:
121.
Auxin is used by gardeners to prepare weed-free lawns. But no damage is caused to grass as auxin
122.
Match List I with List II:
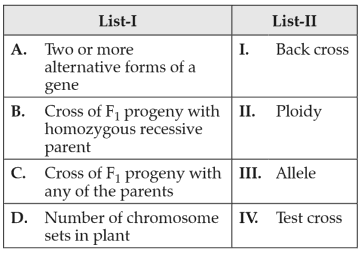
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
123.
Spindle fibres attach to kinetochores of chromosomes during -
124.
Which one of the following can be explained on the basis of Mendel's law of Dominance?
A. Out of one pair of factors one is dominant and the other is recessive.
B. Alleles do not show any expression and both the characters appear as such in F2 generation.
C. Factors occur in pairs in normal diploid plants.
D. The discrete unit controlling a particular character is called factor.
E. The expression of only one of the parental characters is found in a monohybrid cross.
Choose the correct answer from the options given below:
A. Out of one pair of factors one is dominant and the other is recessive.
B. Alleles do not show any expression and both the characters appear as such in F2 generation.
C. Factors occur in pairs in normal diploid plants.
D. The discrete unit controlling a particular character is called factor.
E. The expression of only one of the parental characters is found in a monohybrid cross.
Choose the correct answer from the options given below:
125.
What is the fate of a piece of DNA carrying only gene of interest which is transferred into an alien organism?
A. The piece of DNA would be able to multiply itself independently in the progeny cells of the organism.
B. It may get integrated into the genome of the recipient.
C. It may multiply and be inherited along with the host DNA.
D. The alien piece of DNA is not an integral part of chromosome.
E. It shows ability to replicate.
Choose the correct answer from the options given below :
A. The piece of DNA would be able to multiply itself independently in the progeny cells of the organism.
B. It may get integrated into the genome of the recipient.
C. It may multiply and be inherited along with the host DNA.
D. The alien piece of DNA is not an integral part of chromosome.
E. It shows ability to replicate.
Choose the correct answer from the options given below :
126.
How many molecules of ATP and NADPH are required for every molecule of CO2 fixed in the Calvin cycle?
127.
In a plant, black seed color (BB/Bb) is dominant over white seed color (bb). In order to find out the genotype of the black color seed plant, with which of the following genotype will you cross it?
128.
Lectin, a small molecular weight organic compound found in living tissues, is an example of:
129.
Match List I with List II:
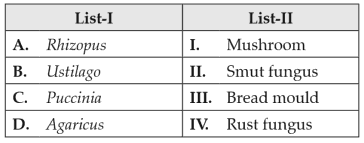

130.
Tropical regions show greatest level of species richness because -
A. Tropical latitudes have remained relatively undisturbed for millions of years, hence more time was available for species diversification.
B. Tropical environments are more seasonal
C. More solar energy is available in tropics
D. Constant environments promote niche specialization.
E. Tropical environments are constant and predictable
Choose the correct answer form the options given below:
A. Tropical latitudes have remained relatively undisturbed for millions of years, hence more time was available for species diversification.
B. Tropical environments are more seasonal
C. More solar energy is available in tropics
D. Constant environments promote niche specialization.
E. Tropical environments are constant and predictable
Choose the correct answer form the options given below:
131.
Match List-I with List-II
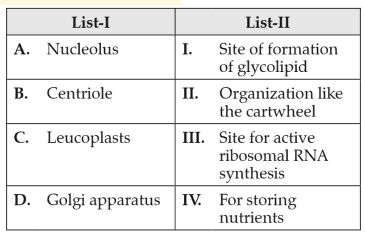
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
132.
The lactose present in the growth medium of bacteria is transported to the cell by the action of
133.
Given below are two statements:
Statement I: Chromosomes become gradually visible under light microscope during leptotene stage.
Statement II: The beginning of diplotene stage is recognized by dissolution of synaptonemal complex.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Chromosomes become gradually visible under light microscope during leptotene stage.
Statement II: The beginning of diplotene stage is recognized by dissolution of synaptonemal complex.
In the light of the above statements, choose the correct answer from the options given below:
134.
Formation of interfascicular cambium from fully developed parenchyma cells is an example for
135.
Given below are two statements:
Statement I: Parenchyma is living but collenchyma is dead tissue.
Statement II: Gymnosperms lack xylem vessels but presence of xylem vessels is the characteristic of angiosperms.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Parenchyma is living but collenchyma is dead tissue.
Statement II: Gymnosperms lack xylem vessels but presence of xylem vessels is the characteristic of angiosperms.
In the light of the above statements, choose the correct answer from the options given below:
136.
Identify the set of correct statements:
A. The flowers of Vallisneria are colourful and produce nectar
B. The flowers of waterlily are not pollinated by water
C. In most of water-pollinated species, the pollen grains are protected from wetting
D. Pollen grains of some hydrophytes are long and ribbon like
E. In some hydrophytes, the pollen grains are carried passively inside water
Choose the correct answer fron the options given below:
A. The flowers of Vallisneria are colourful and produce nectar
B. The flowers of waterlily are not pollinated by water
C. In most of water-pollinated species, the pollen grains are protected from wetting
D. Pollen grains of some hydrophytes are long and ribbon like
E. In some hydrophytes, the pollen grains are carried passively inside water
Choose the correct answer fron the options given below:
137.
Which of the following is an example of actinomorphic flower?
138.
The capacity to generate a whole plant from any cell of the plant is called
139.
Given below are two statements:
Statement I: Bt toxins are insect group specific and coded by a gene cry IAc.
Statement II: Bt toxin exists as inactive protoxin in B. thuringiensis. However, after ingestion by the insect the inactive protoxin gets converted into active form due to acidic pH of the insect gut.
In the light of the above statements, choose the correct answer from the options given below:
Statement I: Bt toxins are insect group specific and coded by a gene cry IAc.
Statement II: Bt toxin exists as inactive protoxin in B. thuringiensis. However, after ingestion by the insect the inactive protoxin gets converted into active form due to acidic pH of the insect gut.
In the light of the above statements, choose the correct answer from the options given below:
140.
List of endangered species was released by-
141.
The cofactor of the enzyme carboxypeptidase is:
142.
Match List I with List II:
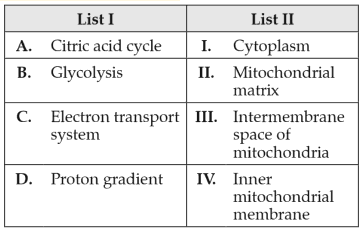
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
143.
Which of the following statement is correct regarding the process of replication in E.coli?
144.
Match List I with List II:

Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
145.
Identify the correct description about the given figure:
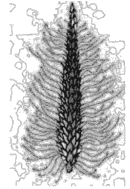

146.
Identify the step in tricarboxylic acid cycle, which does not involve oxidation of substrate.
147.
Given below are two statements:
Statement I: In C3 plants, some O2 binds to RuBisCO, hence CO2 fixation is decreased.
Statement II: In C4 plants, mesophyll cells show very little photorespiration while bundle sheath cells do not show photorespiration
. In the light of the above statements, choose the correct answer from the options given below:
Statement I: In C3 plants, some O2 binds to RuBisCO, hence CO2 fixation is decreased.
Statement II: In C4 plants, mesophyll cells show very little photorespiration while bundle sheath cells do not show photorespiration
. In the light of the above statements, choose the correct answer from the options given below:
148.
In an ecosystem if the Net Primary Productivity (NPP) of first trophic level is 100x(kcal m-2)yr-1, what would be the GPP (Gross Primary Productivity) of the third trophic level of the same ecosystem?
149.
Match List I with List II:
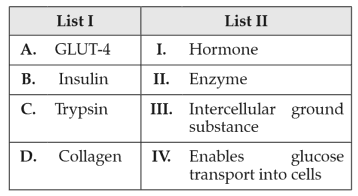
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
150.
Match List I with List II:
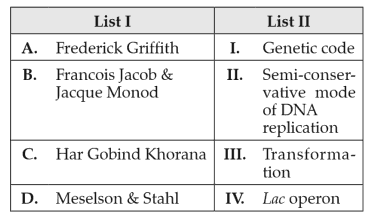 Choose the correct answer from the options given below:
Choose the correct answer from the options given below:
 Choose the correct answer from the options given below:
Choose the correct answer from the options given below:151.
The DNA present in chloroplast is:
152.
Spraying sugarcane crop with which of the following plant growth regulators, increases the length of stem, thus, increasing the yield?
153.
Read the following statements and choose the set of correct statements:
In the members of Phaeophyceae.
A. Asexual reproduction occurs usually by biflagellate zoospores.
B. Sexual reproduction is by oogamous method only.
C. Stored food is in the form of carbohydrates which is either mannitol or laminarin.
D. The major pigments found are chlorophyll a, c and carotenoids and xanthophyll.
E. Vegetative cells have a cellulosic wall, usually covered on the outside by gelatinous coating of algin.
Choose the correct answer from the options given below:
In the members of Phaeophyceae.
A. Asexual reproduction occurs usually by biflagellate zoospores.
B. Sexual reproduction is by oogamous method only.
C. Stored food is in the form of carbohydrates which is either mannitol or laminarin.
D. The major pigments found are chlorophyll a, c and carotenoids and xanthophyll.
E. Vegetative cells have a cellulosic wall, usually covered on the outside by gelatinous coating of algin.
Choose the correct answer from the options given below:
154.
Which of the following are fused in somatic hybridization involving two varieties of plants?
155.
Match List I with List II:
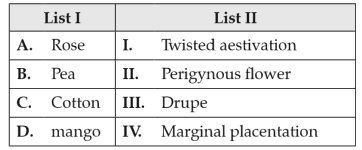
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
156.
Match List I with List II:
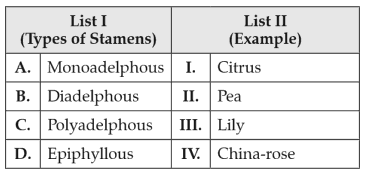 Choose the correct answer from the options given below:
Choose the correct answer from the options given below:
 Choose the correct answer from the options given below:
Choose the correct answer from the options given below:157.
Match List I with List II:
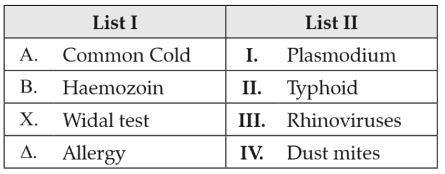
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
158.
The flippers of the penguins and dolphins are the example of the
159.
Given below are some stages of human evolution. Arrange them in correct sequence (Past to recent)
A. Homo habilis
B. Homo sapiens
C. Homo neanderthalensis
D. Homo erectus
Choose the correct sequence of human evolution from the options given below:
A. Homo habilis
B. Homo sapiens
C. Homo neanderthalensis
D. Homo erectus
Choose the correct sequence of human evolution from the options given below:
160.
Which one of the following factors will not affect the Hardy-Weinberg equilibrium?
161.
Which of the following factors are favourable for
the formation of oxyhaemoglobin in alveoli?
162.
Which of the following is not a natural/traditional
contraceptive method?
163.
Match List I with List II:
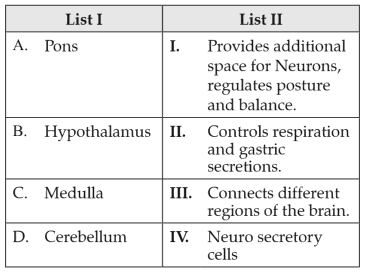
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
164.
Given below are two staements:
Statement I: The presence or absence of hymen is not a reliable indicator of virginity.
Statement II: The hymen is torn during the first coitus only.
In the light of the above staements, choose the correct answer from the options given below:
Statement I: The presence or absence of hymen is not a reliable indicator of virginity.
Statement II: The hymen is torn during the first coitus only.
In the light of the above staements, choose the correct answer from the options given below:
165.
Match List I with List II: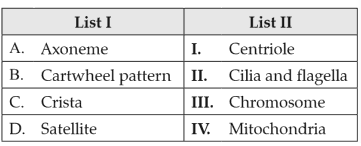
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
166.
Match List I with List II: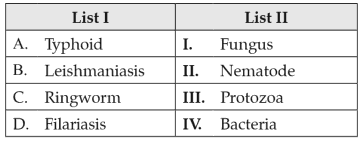
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
167.
Given below are two statements:
Statement I : In the nephron , the descending limb of loop of Henle is impermeable to water and permeable to electrolytes
Statement II : The proximal convoluted tubule is lined by simple columnar brush border epithelium and increases the surface area for reabsorption .
In the light of the above statements , choose the correct answer from the options given below :
Statement I : In the nephron , the descending limb of loop of Henle is impermeable to water and permeable to electrolytes
Statement II : The proximal convoluted tubule is lined by simple columnar brush border epithelium and increases the surface area for reabsorption .
In the light of the above statements , choose the correct answer from the options given below :
168.
Match List I with List II: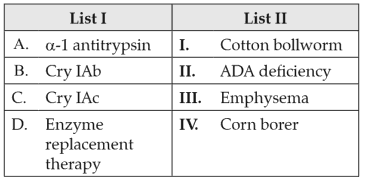
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
169.
Match List I with List II: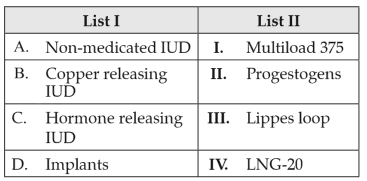
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
170.
Consider the following statements :
A. Annelids are true coelomates
B. Poriferans are pseudocoelomates
C. Aschelminthes are acoelomates
D. Platyhelminthes are pseudocoelomates
Choose the correct answer from the options given below :
A. Annelids are true coelomates
B. Poriferans are pseudocoelomates
C. Aschelminthes are acoelomates
D. Platyhelminthes are pseudocoelomates
Choose the correct answer from the options given below :
171.
Match List I with List II: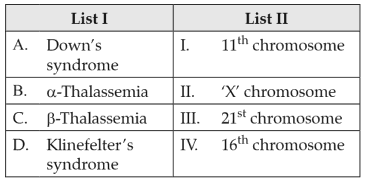
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
172.
Following are the stages of pathway for conduction of an action potential through the heart:
A. AV bundle
B. Purkinje fibres
C. AV node
D. Bundle branches
E. SA node
Choose the correct sequence of pathway from the options given below:
A. AV bundle
B. Purkinje fibres
C. AV node
D. Bundle branches
E. SA node
Choose the correct sequence of pathway from the options given below:
173.
Match List I with List II: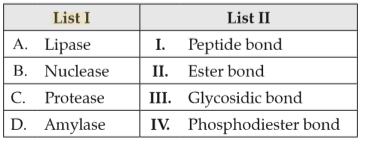
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
174.
The following diagram showing restriction sites in E.coli cloning vector pBR322. Find the role of 'X' and 'Y' genes:
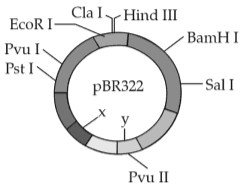

175.
The "Ti plasmid" of Agrobacterium tumefaciens stand for
176.
Match List I with List II: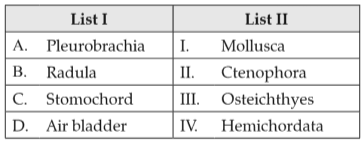
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
177.
Which of the following statements is incorrect?
178.
Which one is the correct product of DNA dependent RNA polymerase to the given template?
3'TACATGGCAAATATCCATTCA5 '
3'TACATGGCAAATATCCATTCA5 '
179.
Match List I with List II: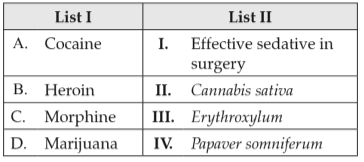
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
180.
Match List I with List II: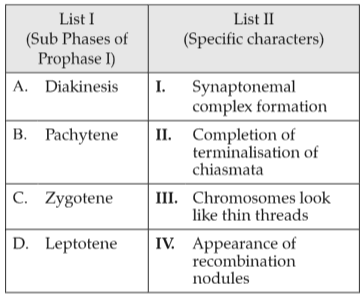
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
181.
In both sexes of cockroach, a pair of jointed filamentous structures called anal cerci are present on:
182.
Given below are two statements : one is labelled as
Assertion A and the other is labelled as Reason R :
Assertion A : Breast - feeding during initial period of infant growth is recommended by doctors for bringing a healthy baby .
Reason R : Colostrum contains several antibodies absolutely essential to develop resistance for the new born baby .
In the light of the above statements , choose the most appropriate answer from the options given below :
Assertion A : Breast - feeding during initial period of infant growth is recommended by doctors for bringing a healthy baby .
Reason R : Colostrum contains several antibodies absolutely essential to develop resistance for the new born baby .
In the light of the above statements , choose the most appropriate answer from the options given below :
183.
Match List I with List II: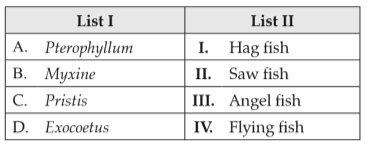
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
184.
Three types of muscles are given as a, b, and c. Identify the correct matching pair along with their location in human body:
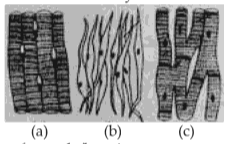
Name of muscle/location

Name of muscle/location
185.
Which of the following is not a steroid hormone?
186.
Match List I with List II: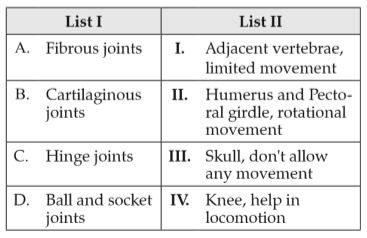
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
187.
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : FSH acts upon ovarian follicles in female and Leydig cells in male .
Reason R : Growing ovarian follicles secrete estrogen in female while interstitial cells secrete androgen in male human being .
In the light of the above statements , choose the correct answer from the options given below :
Assertion A : FSH acts upon ovarian follicles in female and Leydig cells in male .
Reason R : Growing ovarian follicles secrete estrogen in female while interstitial cells secrete androgen in male human being .
In the light of the above statements , choose the correct answer from the options given below :
188.
Match List I with List II: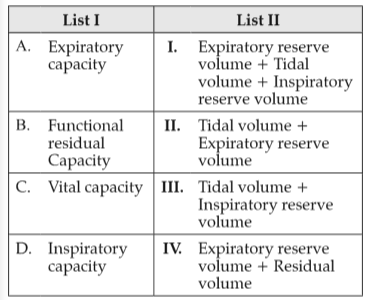
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
189.
Following are the stages of cell division:
A. Gap 2 phase
B. Cytokinesis
C. Synthesis phase
D. Karyokinesis
E. Gap 1 phase
Choose the correct sequence of stages from the options given below:
A. Gap 2 phase
B. Cytokinesis
C. Synthesis phase
D. Karyokinesis
E. Gap 1 phase
Choose the correct sequence of stages from the options given below:
190.
Which of the following are Autoimmune disorders?
A. Myasthenia gravis
B. Rheumatoid arthritis
C. Gout
D. Muscular dystrophy
E. Systemic Lupus Erythmatosus(SLE)
Choose the most appropriate answer from the options given below:
A. Myasthenia gravis
B. Rheumatoid arthritis
C. Gout
D. Muscular dystrophy
E. Systemic Lupus Erythmatosus(SLE)
Choose the most appropriate answer from the options given below:
191.
Which of the following is not a component of Fallopian tube?
192.
As per ABO blood grouping system, the blood group of father is B+, mother is A+ and child is O+.
Their respective gentype can be
A. IBi / IAi / ii
B. IBIB / IAIAI / ii
C. IAIB /i IAI /IBi
D. IAi / IBi /IAi
E. iIB / iIA / IAIB
Choose the most appropriate answer from the options given below:
Their respective gentype can be
A. IBi / IAi / ii
B. IBIB / IAIAI / ii
C. IAIB /i IAI /IBi
D. IAi / IBi /IAi
E. iIB / iIA / IAIB
Choose the most appropriate answer from the options given below:
193.
Match List I with List II: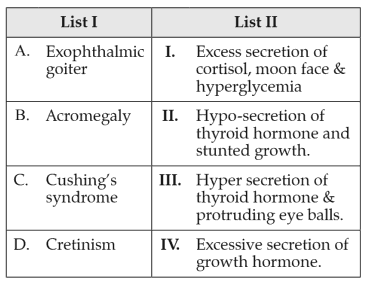
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
194.
Match List I with List II: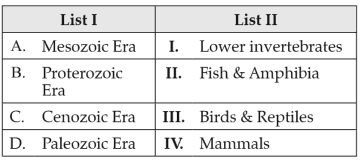
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
195.
The following are the statements about non-chordates:
A. Pharynx is perforated by gill slits.
B. Notochord is absent.
C. Central nervous system is dorsal.
D. Heart is dorsal if present.
E. Post anal tail is absent.
Choose the most appropriate answer from the options given below :
A. Pharynx is perforated by gill slits.
B. Notochord is absent.
C. Central nervous system is dorsal.
D. Heart is dorsal if present.
E. Post anal tail is absent.
Choose the most appropriate answer from the options given below :
196.
Match List I with List II: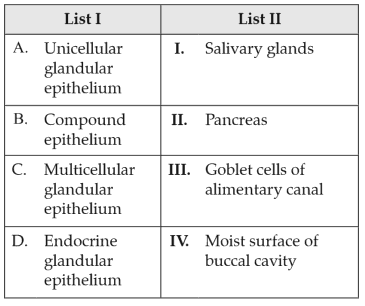
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
197.
Match List I with List II: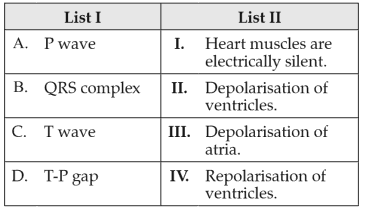
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
198.
Given below are two statements:
Statement I : Mitochindria and chloroplasts are both double membrane bound organelles.
Statement II: Inner memebrane of mitochondria is relatively less permeable, as compared to chroloroplast.
In the light of the above staements, choose the most appropriate answer from the options given below
Statement I : Mitochindria and chloroplasts are both double membrane bound organelles.
Statement II: Inner memebrane of mitochondria is relatively less permeable, as compared to chroloroplast.
In the light of the above staements, choose the most appropriate answer from the options given below
199.
Match List I with List II: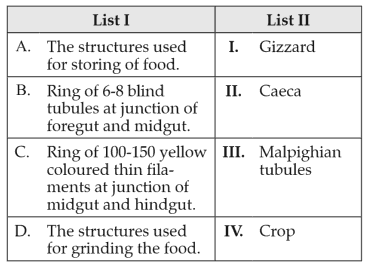
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
200.
Match List I with List II: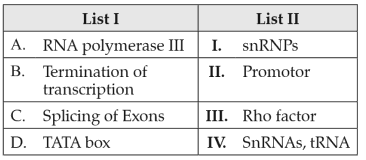
Choose the correct answer from the options given below:

Choose the correct answer from the options given below: