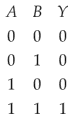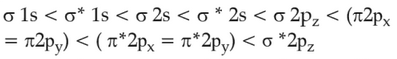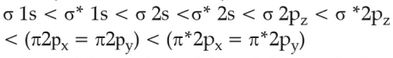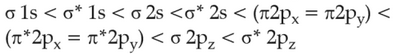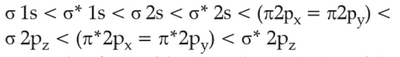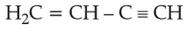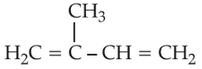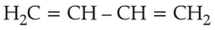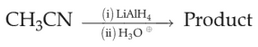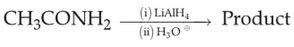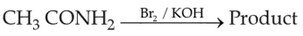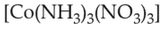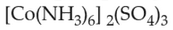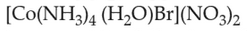Home >> NEET - 2023
Welcome and Shine in NEET with Shine NEET SmartPrep!.
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Please register for a Free trial. You would be able to try a sub set of these questions in your free trial. We recommend you explore the full system and go for a one time subscription if you are satisfied.
Try it out and feel the difference!!
Register
Your Full Test Performance Summary
Questions Available: 200
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
1.
The angular acceleration of a body, moving along the circumference of a circle, is
2.
A Carnot engine has an efficiency of 50% when its source is at a temperature \(327^\circ C\). The temperature of the sink is
3.
A bullet from a gun is fired on a rectangular wooden block with velocity u. When bullet travels 24 cm through the block along its length horizontally, velocity of bullet becomes u/3. Then it further penetrates into the block in the same direction before coming to rest exactly at the other end of the block. The total length of the block is
4.
Let a wire be suspended from the ceiling (rigid support) and stretched by a weight W attached at its free end. The longitudinal stress at anypoint of cross-sectional area A of the wire is
5.
Given below are two statements:
Statement I:Photovoltaic devices can convert optical radiation into electricity.
Statement II:Zener diode is designed to operate under reverse bias in breakdown region. In the light of the above statements, choose the most appropriate answer from the options given below.
Statement I:Photovoltaic devices can convert optical radiation into electricity.
Statement II:Zener diode is designed to operate under reverse bias in breakdown region. In the light of the above statements, choose the most appropriate answer from the options given below.
6.
A full wave rectifier circuit consists of two p-n junction diodes, a centre-tapped transformer, capacitor and a load resistance. Which of these components remove the ac ripple from the rectified output?
7.
The net magnetic flux through any closed surface is
8.
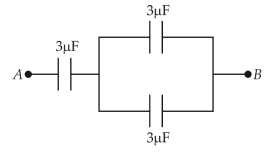
The equivalent capacitance of the system shown in the following circuitis

9.
A football player is moving southward and suddenly turns eastward with the same speed to avoid an opponent. The force that acts on the player while turning is
10.
The net impedence of circuit (as shown in figure) will be


11.
An ac source is connected to a capacitor C. Due to decrease in itsoperating frequency
12.
The potential energy of a long spring when stretched by 2cm is U. If the spring is stretched by 8 cm, potential energy stored in it will be
13.
Two bodies of mass \(m\) and \(9m\) are placed at a distance \(R\). The gravitational potential on the line joining the bodies where the gravitational field equals zero, will be ( \(G = gravitational\, constant\))
14.
A satellite is orbiting just above the surface of the earth with period \(T\). If \(d\) is the density of the earth and \(G\) is the universal constant of gravitation, the quantity \(\displaystyle \frac{3\pi}{Gd}\) represents
15.
The errors in the measurement which arise due to unpredictable fluctuations in temperature and voltage supply are
16.
A metal wire has mass (0.4 ± 0.002) g, radius (0.3 ± 0.001) mm and length (5 ± 0.02) cm. The maximum possible percentage error in the measurement of density will nearly be
17.
The ratio of radius of gyration of a solid sphere of mass M and radius R about its own axis to the radius of gyration of the thin hollow sphere of same mass and radius about its axis is
18.
The temperature of a gas is \(−50^\circ C\). To what temperature the gas should be heated so that the rms speed is increased by 3 times?
19.
A vehicle travels half the distance with speed v and the remaining distance with speed 2v. Its average speed is
20.
A horizontal bridge is built across a river. A student standing on the bridge throws a small ball vertically upwards with a velocity 4 ms−1. The ball strikes the water surface after 4 s. The height of bridge above water surface is (Take g = 10m s−2)
21.
The work functions of Caesium (Cs), Potassium (K) and Sodium (Na)are 2.14eV, 2.30eV and 2.75eV respectively. If incident electromagneticradiation has an incident energy of 2.20eV, which of thesephotosensitive surfaces may emit photoelectrons?
22.
The amount of energy required to form a soap bubble of radius 2 cm from a soap solution is nearly (surface tension of soap solution = 0.03 Nm−1)
23.
The venturi-meter works on
24.
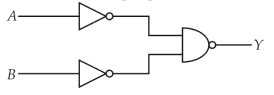
For the following logic circuit, the truth table is

25.
The magnetic energy stored in an inductor of inductance 4μH carrying a current of 2A is
26.
In a series LCR circuit, the inductance L is 10mH,capacitance C is 1μF and resistance R is 100Ω. The frequency at which resonance occurs is
27.
A 12V, 60W lamp is connected to the secondary of a step-down transformer, whose primary is connected to ac mains of 220V. Assuming the transformer to be ideal, what is the current in the primary winding?
28.
A bullet is fired from a gun at the speed of 280 cm\( s^{−1} \) in the direction \( 30^\circ \) above the horizontal. The maximum height attained by the bullet is (\( g = 9.8 ms^{−2} \), \( Sin30^\circ \) = 0.5)
29.
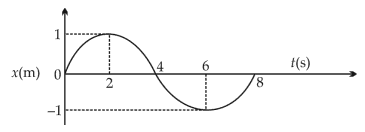
The x-t graph of a particle performing simple harmonic motion is shown in the figure. The acceleration of the particle at \(t = 2s\) is

30. 

31.
An electric dipole is placed at an angle of \(30^\circ\) with an electric field of intensity \(2 × 10^5NC^{−1}\). It experiences a torque equal to 4 Nm. Calculate the magnitude of charge on the dipole, if the dipole length is 2 cm.
32.
An electric dipole is placed as shown in the figure.
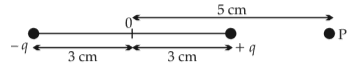
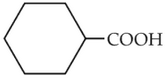
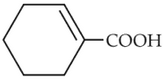
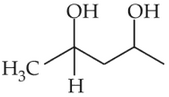
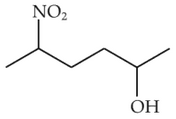
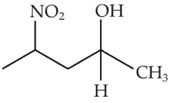
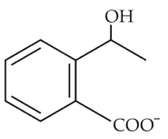
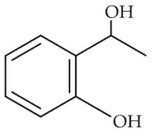
The electric potential in \(10^2\,V\) at point P due to dipole is (\(E_0 =\).permittivity of free space and \(\displaystyle \frac{1}{4πϵ_0}=k\) )

The electric potential in \(10^2\,V\) at point P due to dipole is (\(E_0 =\).permittivity of free space and \(\displaystyle \frac{1}{4πϵ_0}=k\) )
33.
The ratio of frequencies of fundamental harmonic produced by an open pipe to that of closed pipe having the same length is
34.
Resistance of a carbon resistor determined from colour codes is (22000±5%) Ω. The colour of third band must be
35.
The magnitude and direction of the current in the following circuit is
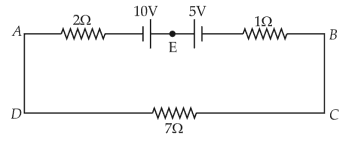

36.
Calculate the maximum acceleration of a moving car so that a body lying on the floor of the car remains stationary. The coefficient of static friction between the body and the floor is 0.15 (g = 10 \(m s^{−2})\).
37.
10 resistors, each of resistance R are connected in series to a battery of
emf E and negligible internal resistance. Then those are connected in
parallel to the same battery, the current is increased n times. The value
of n is
38.
The resistance of platinum wire at \(0^\circ C\) is \(2 \Omega\) and \(6.8 \Omega\) at \(80^ \circ C\). The temperature coefficient of resistance of the wire is
39.
The half life of a radioactive substance is 20 minutes. In how much time, the activity of substance drops to \(\displaystyle \left(\frac{1}{16}\right)^{th}\) of its initial value?
40.
In hydrogen spectrum, the shortest wavelength in the Balmer series is \(\lambda\). The shortest wavelength in the Bracket series is
41.
The radius of inner most orbit of hydrogen atom is \(\displaystyle 5.3 \times 10^{-11} \) m. What is the radius of third allowed orbit of hydrogen atom?
42.
In a plane electromagnetic wave travelling in free space, the electric field component oscillates sinusoidally at a frequency of \(2.0 \times10^{10}\) Hz and amplitude \(48\,Vm^{−1}\). Then the amplitude of oscillating magnetic field is (Speed of light in free space = \( 3 \times 10^8\, ms^{−1}\))
43.
The minimum wavelength of X-rays produced by an electron acceleratedthrough a potential difference of V volts is proportional to
44.
If the galvanometer G does not show any deflection in the circuit shown, the value of R is given by
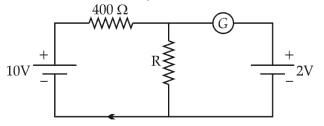

45.
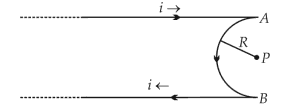
A very long conducting wire is bent in a semi-circular shape from A to B as shown in figure. The magnetic field at point P for steady current configuration is given by

46.
A wire carrying a current I along the positive x-axis has length L. It is kept in a magnetic field \(\vec{B}\) = \(\left( 2\hat{i} + 3\hat{j} - 4\hat{j}\right)\)T. The magnitude of the magnetic force acting on the wire is
47.
Light travels a distance x in time t1 in air and 10x in time t2 in another denser medium. What is the critical angle for this medium?
48.
For Young's double slit experiment, two statements are given below:
Statement I : If screen is moved away from the plane of slits, angular separation of the fringes remains constant.
Statement II : If the monochromatic source is replaced by another monochromatic source of larger wavelength, the angular separation of fringes decreases.
In the light of the above statements, choose the correct answer from the options given below :
Statement I : If screen is moved away from the plane of slits, angular separation of the fringes remains constant.
Statement II : If the monochromatic source is replaced by another monochromatic source of larger wavelength, the angular separation of fringes decreases.
In the light of the above statements, choose the correct answer from the options given below :
49.
In the figure shown here, what is the equivalent focal length of the combination of lenses (Assume that all layers are thin) ?
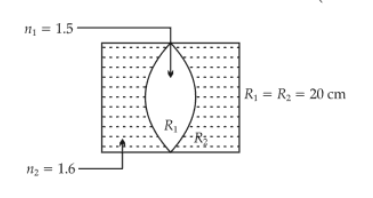

50.
Two thin lenses are of same focal lengths (F), but one is convex and the other one is concave. When they are placed in contact with each other, the equivalent focal length of the combination will be :
51.
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
52.
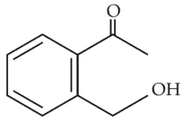
Identify product (A) in the following reaction:


53.
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R :
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2
In the light of the above statements, choose the correct answer from the options given below:
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2
In the light of the above statements, choose the correct answer from the options given below:
54.
9. Match List-I with List-II.
Choose the correct answer from the options
given below:
| List-I | List-II |
|---|---|
| A. Coke | I. Carbon atoms are sp3 hybridised |
| B. Diamond | II. Used as a dry lubricant |
| C. Fullerene | II. Used as a reducing Oagent |
| D. Graphite | IV. Cage like molecules |
55.
The conductivity of centimolar solution of KCl at
\(25^\circ C\) is 0.0210 ohm-1 and cm-1 and the resistance
of the cell containing the solution at \(25^\circ C\) is 60 ohm. The value of cell constant is-
56.
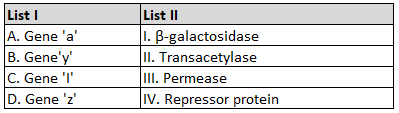
For a certain reaction, the rate = k [A]2 [B], when the initial concentration of A is tripled keeping concentration of B constant, the initial rate would
57.
Which one is an example of heterogeneous catalysis?
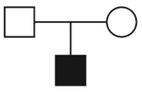
58.
Amongst the following, the total number of
species NƠT having eight electrons around central
atom in its outer most shell, is
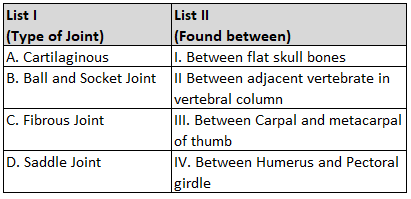
NH3, AICI3, BeCl2, CCl4, PCI 5 :
59.
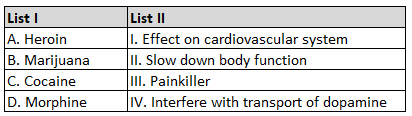
The correct order of energies of molecular orbitals
of N2 molecule, is
60.
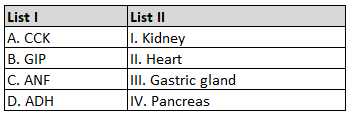
The number of \(\sigma\) bonds, \(\pi\) bonds and lone pair of electrons in pyridine, respectively are:
61.
The element expected to form largest ion to achieve the nearest noble gas configuration is
62.
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R :
Assertion A : A reaction can have zero activation energy.
Reason R: The minimum extra amount ofenergy absorbed by reactant molecules so that their enengy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : A reaction can have zero activation energy.
Reason R: The minimum extra amount ofenergy absorbed by reactant molecules so that their enengy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
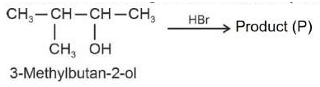
63.
Consider the following reaction and identify the
product (P).

64.
Given below are two statements : one is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : In equation \(\delta_r\, G\, =\, -nFE_{\text{cell}}\), value of \(\delta_r\, G\) depends on n.
Reason R: \(E_{\text{cell}}\) is an intensive property and \(\delta_r\, G\) is an extensive property.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : In equation \(\delta_r\, G\, =\, -nFE_{\text{cell}}\), value of \(\delta_r\, G\) depends on n.
Reason R: \(E_{\text{cell}}\) is an intensive property and \(\delta_r\, G\) is an extensive property.
In the light of the above statements, choose the correct answer from the options given below:
65.
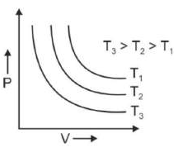
Which amonst the following options is correct graphical representation of Boyle's law?
66.
In Lassaigne's extract of an organic compound, both nitrogen and sulphur are present, which gives blood red colour with Fe3+ due to the formation of
67.
Identify the product in the following reaction:
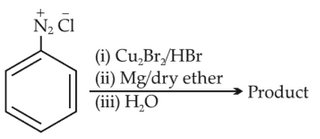
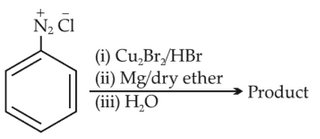
68.
Select the correct Statements from the following:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
69.
A compound is formed by two elements A and B.The elements B forms cubic close packed structure and atoms of A occupy \(\frac{1}{3}\) of tetrahedralvoids. If the formula of the compound is AxBy, thenthe value of x + y is in option.
70.
Given below are two statements:
Statemnent I : A unit formed by the attachment of a base of 1' position of sugar is known as nucleoside.
Statement II : When nucleoside is linked to phosphorous acid at 5-position of sugar moiety, we get nucleotide.
In the light of the above statements, choose the correct answer from the options given below:
Statemnent I : A unit formed by the attachment of a base of 1' position of sugar is known as nucleoside.
Statement II : When nucleoside is linked to phosphorous acid at 5-position of sugar moiety, we get nucleotide.
In the light of the above statements, choose the correct answer from the options given below:
71.
Which amongst the following molecules on
polymerization produces neoprene?
72.
Taking stability as the factor, which one of the
following represents correct relationship?
73.
Some tranquilizers are listed below. Which onefrom the following belongs to barbiturates?
74.
Which of the following statements are NOTcorrect?
A. Hydrogen is used to reduce heavy metal oxidesto metals.
B. Heavy water is used to study reactionmechanism.
C. Hydrogen is used to make saturated fats formoils.
D. The H-H bond dissociation enthalpy is lowestas compared to a single bond between twoatoms of any element.
E. Hydrogen reduces oxides of metals that aremore active than iron.
Choose the most approriate answer from theoptions given below:
A. Hydrogen is used to reduce heavy metal oxidesto metals.
B. Heavy water is used to study reactionmechanism.
C. Hydrogen is used to make saturated fats formoils.
D. The H-H bond dissociation enthalpy is lowestas compared to a single bond between twoatoms of any element.
E. Hydrogen reduces oxides of metals that aremore active than iron.
Choose the most approriate answer from theoptions given below:
75.
Intermolecular forces are forces of attraction andrepulsion between interacting particles that willinclude:
A. dipole - dipole forces.
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from theoptions given below:
A. dipole - dipole forces.
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from theoptions given below:
76.
Amonst the given options which of the following
molecules/ion acts as a Lewis acid?
77.
The right option for the mass of CO2 produced by heating 20g of 20% pure limestone is
(Atomic mass of Ca = 40)
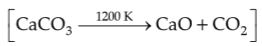

78.
The relation between nm, (nm = the number of permissible values of magentic quantum number (m)) for a given value of aziuthal quantum number (l), is
79.
The stability of Cu is more than Cu+ salts in aqueous solution due to
80.
Which one of the following statements is correct?
81.
Which of the following reactions will NOT give
primary amine as the product?
82.
The given compound
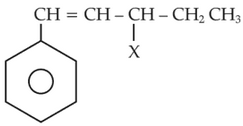
is an example of

is an example of
83.
Complete the following reaction:


84.
Homoleptic complex from the following complex
is:
85.
Weight (g) of two moles of the organic compound,
which is obtained by heating sodium ethanoate
with sodium hydroxide in presence of calcium
Oxide is:
86.
Consider the following reaction
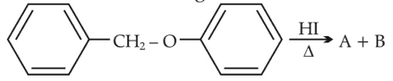 Identify products A and B:-
Identify products A and B:-
 Identify products A and B:-
Identify products A and B:-87.
Which amongst the following will be most readily
dehydrated under acidic conditions?
88.
The equilibrium concentrations of the species in the reaction \(A\,+\,B\, \rightleftharpoons\, C \,+\,D\) are 2, 3, 10, and 6 mol L-1, respectively at 300 K. \(\delta G^\circ\) for the reaction is (R = 2 cal/mol K).
89.
Given below are two statements :
Statement I: The nutrient deficient water bodies lead to eutrophication.
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose thecorrect answer from the options given below:
Statement I: The nutrient deficient water bodies lead to eutrophication.
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose thecorrect answer from the options given below:
90.
Which amongst the following options is the correct
relation between change in enthalpy and change
in internal energy?
91.
Match List-I with List-II
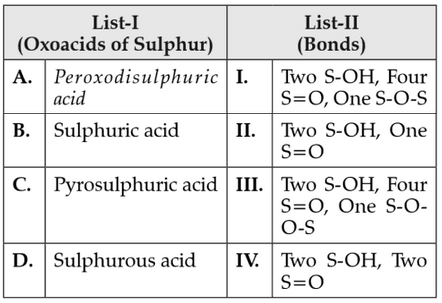
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
92.
Identify the major product obtained in the
following reaction:
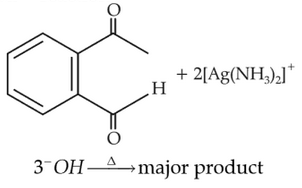

93.
Pumice stone is an example of-
94.
The reaction that does NOT take place in blastfurnace between 900 K to 1500 K temperaturerange during extraction of iron is:
95.
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
96.
Consider the following compounds/species.
The number of compounds/species which obey Huckel's rule is
The number of compounds/species which obey Huckel's rule is
97.
What fraction of one edge centred octahedral voidlies in one unit cell of fcc?
98.
Which complex compound is most stable?
99.
On balancing the given redox reaction,
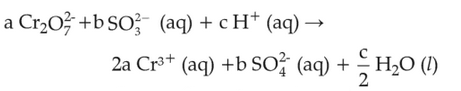
the coefficients a, b and c are found to be, respectively
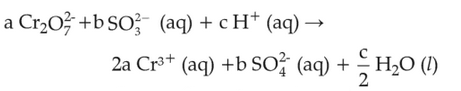
the coefficients a, b and c are found to be, respectively
100.
Identify the final product [D] obtained in the
following sequence of reactions.


101.
Given below are two statements:
Statement I : The forces generated by transpiration can lift a xylem-sized column of water over 130 meters height.
Statement II : Transpiration cools leaf surfaces sometimes 10 to 15 degrees, by evaporative cooling.
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement I : The forces generated by transpiration can lift a xylem-sized column of water over 130 meters height.
Statement II : Transpiration cools leaf surfaces sometimes 10 to 15 degrees, by evaporative cooling.
In the light of the above statements, choose the most appropriate answer from the options given below:
102.
Given below are two statements : One is labelled
as Assertion A and the other is labelled as Reason R.
Assertion A : The first stage of gametophyte in the life cycle of moss is protonema stage.
Reason R: Protonema develops directly from spores produced in capsule.
In the light of the above statements, choose the most appropriate answer from the options given below:
Assertion A : The first stage of gametophyte in the life cycle of moss is protonema stage.
Reason R: Protonema develops directly from spores produced in capsule.
In the light of the above statements, choose the most appropriate answer from the options given below:
103.
Identify the correct statements:
A. Lenticels are the lens-shaped openings permitting the exchange of gases.
B. Bark formed early in the season is called hard bark.
C. Bark is a technical term that refers to all tissues exterior to vascular cambium.
D. Bark refers to periderm and secondary phloem.
E. Phellogen is single-layered in thickness.
Choose the correct answer fromn the options given below:
A. Lenticels are the lens-shaped openings permitting the exchange of gases.
B. Bark formed early in the season is called hard bark.
C. Bark is a technical term that refers to all tissues exterior to vascular cambium.
D. Bark refers to periderm and secondary phloem.
E. Phellogen is single-layered in thickness.
Choose the correct answer fromn the options given below:
104.
Which of the following statements are correct
about Klinefelter's Syndrome?
A. This disorder was first described by Langdon Down (1866).
B. Such an individual has overall masculine development. However, the feminine development is also expressed.
C. The affected individual is short statured.
D. Physical, psychomotor and mental development is retarded.
E. Such individuals are sterile.
Choose the correct answer from the options given below:
A. This disorder was first described by Langdon Down (1866).
B. Such an individual has overall masculine development. However, the feminine development is also expressed.
C. The affected individual is short statured.
D. Physical, psychomotor and mental development is retarded.
E. Such individuals are sterile.
Choose the correct answer from the options given below:
105.
Given below are two statements : One is labelled
as Assertion A and the other is labelled as
Reason R.
Assertion A: In gymnosperms the pollen grains are released from the microsporangium and carried by air currents.
Reason R: Air currents carry the pollen grains to the mouth of the archegonia where the male gametes are discharged and pollen tube is not formed.
In the light of the above statemnents, choose the correct answer from the options given below:
Assertion A: In gymnosperms the pollen grains are released from the microsporangium and carried by air currents.
Reason R: Air currents carry the pollen grains to the mouth of the archegonia where the male gametes are discharged and pollen tube is not formed.
In the light of the above statemnents, choose the correct answer from the options given below:
106.
Match List I with List II.

Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
107.
Match List I with List II

Choose the correct answer from the options give below:

Choose the correct answer from the options give below:
108.
Given below are two statements:
Statement I: In prokaryotes, the positively charged DNA is held with some negatively charged proteins in a region called nucleoid.
Statemnent II : In eukaryotes, the negatively charged DNA is wrapped around the positively charged histone octamer to form nucleosome.
In the light of the above statements, choose the correct answer from the options given below
Statement I: In prokaryotes, the positively charged DNA is held with some negatively charged proteins in a region called nucleoid.
Statemnent II : In eukaryotes, the negatively charged DNA is wrapped around the positively charged histone octamer to form nucleosome.
In the light of the above statements, choose the correct answer from the options given below
109.
Select the correct statements.
A. Tetrad formation is seen during Leptotene.
B. During Anaphase, the centromeres split and chromatide separate.
C. Terminalization takes place during Pachytene.
D. Nucleolus, Golgi complex and ER are reformed during Telophase.
E. Crossing over takes place between sister chromatids of homologous chromosome.
Choose the correct answer from the options given below:
A. Tetrad formation is seen during Leptotene.
B. During Anaphase, the centromeres split and chromatide separate.
C. Terminalization takes place during Pachytene.
D. Nucleolus, Golgi complex and ER are reformed during Telophase.
E. Crossing over takes place between sister chromatids of homologous chromosome.
Choose the correct answer from the options given below:
110.
Movement and accumulation of ions across a membrane against their concentration gradient can be explained by
111.
Among "The Evil Quartet', which one is considered
the most important cause driving extinetion of
species?
112.
Identify the pair of heterosporous pteridophytes
among the following:
113.
Frequency of recombination between gene pairs
on same chromosome as a measure of the distance
between genes to map their position on
chromosome, was used for the first tìme by
114.
What is the function of tassels in the corn cob?
115.
Identify the correct statements:
A. Detrivores perform fragmentation.
B. The humus is further degraded by some microbes during mineralization.
C. Water soluble inorganic nutrients go down into the soil and get precipitated by a process called leaching.
D. The detritus food chain begins with living organisms.
E. Earthworms break down detritus into smaller particles by a process called catabolism.
Choose the correct answer from the option given below :
A. Detrivores perform fragmentation.
B. The humus is further degraded by some microbes during mineralization.
C. Water soluble inorganic nutrients go down into the soil and get precipitated by a process called leaching.
D. The detritus food chain begins with living organisms.
E. Earthworms break down detritus into smaller particles by a process called catabolism.
Choose the correct answer from the option given below :
116.
Given below are two statements: One is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : Late wood has fewer xylary elements with narrow vessels.
Reason R: Cambium is less active in winters.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : Late wood has fewer xylary elements with narrow vessels.
Reason R: Cambium is less active in winters.
In the light of the above statements, choose the correct answer from the options given below:
117.
The process of appearance of recombination
nodules occurs at which sub stage of prophase I in
meiosis ?
118.
Which of the following stages of meiosis involves
division of centromere?
119.
During the purification process for recombinant
DNA technology, addition of chilled ethanol
precipitates out
120.
Family Fabaceae differs from Solanaceae and
Liliaceae. With respect to the stamens, pick out
the characteristics specific to family Fabaceae but
not found in Solanaceae or Liliaceae.
121.
Large, colourful, fragrant flowers with nectar are
seen in
122.
Spraying of which of the following phytohormone
or juvenile conifers helps in hastening the maturity
period, that leads to early seed production?
123.
Axile placentation is observed in
124.
Among eukaryotes, replication of DNA takes place
in:
125.
How many ATP and NADPH2 are required for the synthesis of one molecule of Glucose during Calvin cycle ?
126.
In gene gun method used to introduce alien DNA
into host cells, microparticles of _______ metal are used.
127.
The thickness of ozone in a column of air in theatmosphere is measured in terms of :
128.
Unequivocal proof that DNA is the genetic
material was first proposed by
129.
In the equation
GPP-R= NPP
GPP is Gross Primary Productivity
NPP is Net Primary Productivity
R here is
GPP-R= NPP
GPP is Gross Primary Productivity
NPP is Net Primary Productivity
R here is
130.
What is the role of RNA polymerase III in the
process of transcription in Eukaryotes ?
131.
Which micronutrient is required for splitting of
water molecule during photosynthesis?
132.
In angiosperm, the haploid, diploid and triploid
structures of a fertilized embryo sac sequentially
are:
133.
Given below are two statements: One is labelled as
Assertion A and the other is labelled as Reason R:
Assertion A : ATP is used at two steps in glycolysis.
Reason R : First ATP is used in converting glucose into glucose-6-phosphate and second ATP is used in conversion of fructose-6-phosphate into fructose-1-6-diphosphate.
In the light of the above statements, choose the correct answer from the options given below :
Assertion A : ATP is used at two steps in glycolysis.
Reason R : First ATP is used in converting glucose into glucose-6-phosphate and second ATP is used in conversion of fructose-6-phosphate into fructose-1-6-diphosphate.
In the light of the above statements, choose the correct answer from the options given below :
134.
Cellulose does not form blue colour with lodine
because:
135.
Which hormone promotes internode/petiole
elongation in deep water rice?
136.
Expressed Sequence Tags (ESTs) refers to:
137.
Upon exposure to UV radiation, DNA stained with ethidium bromide will show
138.
The historic Convention on Biological Diversity,
The Earth Summit' was held in Rio de Janeiro in
the year:
139.
The reaction centre in PS II has an absorption
maxima at
140.
In tissue culture experiments, leaf mesophyll cells
are put in a culture medium to form callus. This
phenomenon may be called as:
141.
Given below are two statements :
Statement I : Endarch and exarch are the terms often used for describing the position of secondary xylem in the plant body.
Statement II : Exarch condition is the most common feature of the root system.
In the light of the above statements, choose the correct answer from the options given below:
Statement I : Endarch and exarch are the terms often used for describing the position of secondary xylem in the plant body.
Statement II : Exarch condition is the most common feature of the root system.
In the light of the above statements, choose the correct answer from the options given below:
142.
Match List I with List II:
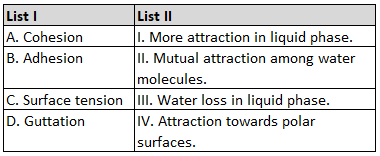
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
143.
Match List I with List II :

Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
144.
Given below are two statements:
Statement I : Gause's 'Competitive Exclusion Principle' states that two closely related species competing for the same resources cannot co-exist indefinitely and competitively inferior one will be eliminated eventually.
Statement II : In general, carnivores are more adversely affected by competition than herbivores.
In the light of the above statements, choose the correct answer from the options given below:
Statement I : Gause's 'Competitive Exclusion Principle' states that two closely related species competing for the same resources cannot co-exist indefinitely and competitively inferior one will be eliminated eventually.
Statement II : In general, carnivores are more adversely affected by competition than herbivores.
In the light of the above statements, choose the correct answer from the options given below:
145.
How many diferent proteins does the ribosome
consist of?
146.
Which of the following combinations is required
for chemiosmosis?
147.
Which one of the following statements is NOT
correct?
148.
Match List I with List II:
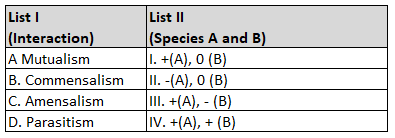
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
149.
Main steps in the formation of Recombinant DNA
are given below. Arrange these steps in a correct
sequence.
A. Insertion of recombinant DNA into the host cell.
B. Cutting of DNA at specific location by restriction enzyme.
C. Isolation of desired DNA fragment.
D. Amplification of gene of interest using PCR.
Choose the correct answer from the options given below:
A. Insertion of recombinant DNA into the host cell.
B. Cutting of DNA at specific location by restriction enzyme.
C. Isolation of desired DNA fragment.
D. Amplification of gene of interest using PCR.
Choose the correct answer from the options given below:
150.
Match List I with List II :
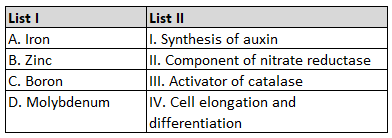
Choose the correct answer from the options given below

Choose the correct answer from the options given below
151.
Match List I with List II:
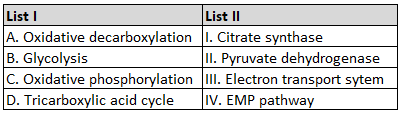
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
152.
Given below are two statemente: One is labelled
as Assertion A and the other is labelled as Reason R.
Assertion: A flower is defined as modified shoot wherein the shoot apical meristem changes to floral meristem.
Reason R: Internode of the shoot gets condensed to produce different floral appendages laterally at successive nodes instead of leaves.
In the light of the above statements, choose the correct answer from the options given below:
Assertion: A flower is defined as modified shoot wherein the shoot apical meristem changes to floral meristem.
Reason R: Internode of the shoot gets condensed to produce different floral appendages laterally at successive nodes instead of leaves.
In the light of the above statements, choose the correct answer from the options given below:
153.
Melonate inhibits the growth of pathogenic
bacteria by inhibiting the activity of
154.
Given below are two statements :
Statement I : A protein is imagined as a line, the left end represented by first amino acid (C-terminal) and the right end represented by last amino acid (N-terminal).
Statement II : Adult human haemoglobin, consists of 4 subunits (two subunits of \(\alpha\) type and two subunits of \(\beta\) type.)
In the light of the above statements, choose the correct answer from the options given below:
Statement I : A protein is imagined as a line, the left end represented by first amino acid (C-terminal) and the right end represented by last amino acid (N-terminal).
Statement II : Adult human haemoglobin, consists of 4 subunits (two subunits of \(\alpha\) type and two subunits of \(\beta\) type.)
In the light of the above statements, choose the correct answer from the options given below:
155.
Radial symmetry is NOT found in adults of phylum _______
156.
Which of the following statements are correct
regarding female reproductive cycle ?
A. In non-primate mammals cyclical changes during reproduction are called oestrus cycle.
B. First menstrual cycle begins at puberty and is called menopause.
C. Lack of menstruation may be indicative of pregnancy.
D. Cyclic menstruation extends between menarche and menopause.
Choose the most appropriate answer from the options given below :
A. In non-primate mammals cyclical changes during reproduction are called oestrus cycle.
B. First menstrual cycle begins at puberty and is called menopause.
C. Lack of menstruation may be indicative of pregnancy.
D. Cyclic menstruation extends between menarche and menopause.
Choose the most appropriate answer from the options given below :
157.
Given below are two statements : one is labelled as
Assertion A and the other is labelled as Reason R
Assertion A: Nephrons are of two types: Cortical & Juxta medullary, based on their relative position in cortex and medulla.
Reason R: Juxta medullary nephrons have short loop of Henle. Whereas, cortical nephrons have longer loop of Henle.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A: Nephrons are of two types: Cortical & Juxta medullary, based on their relative position in cortex and medulla.
Reason R: Juxta medullary nephrons have short loop of Henle. Whereas, cortical nephrons have longer loop of Henle.
In the light of the above statements, choose the correct answer from the options given below:
158.
Match List I with List II with respect to human
eye.
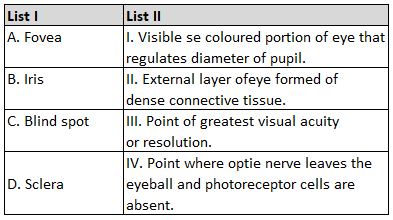
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
159.
Which of the following are NOT considered as the
part of endomembrane system
A. Mitochondria
B. Endoplasmic Reticulum
C. Chloroplasts
D. Golgi complex
E. Peroxisomes
Choose the most appropriate answer from the options given below :
A. Mitochondria
B. Endoplasmic Reticulum
C. Chloroplasts
D. Golgi complex
E. Peroxisomes
Choose the most appropriate answer from the options given below :
160.
Broad palm with single palm crease is visible in a
person suffering from:
161.
Match List I with List II.

Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
162.
Which one of the following common sexually
transmitted diseases is completely curable when
detected early and treated properly ?
163.
Given below are two statements : one is labelled
as Assertion A and the other is labelled as
Reason R.
Assertion A : Endometrium is necessary for implantation of blastocyst.
Reason R: In the absence of fertilization, the corpus luteum degenerates that causes disintegration of endometrium.
In the light of the above statements, choose the correct answer from the options given below:
Assertion A : Endometrium is necessary for implantation of blastocyst.
Reason R: In the absence of fertilization, the corpus luteum degenerates that causes disintegration of endometrium.
In the light of the above statements, choose the correct answer from the options given below:
164.
Which of the following is not a cloning vector ?
165.
Given below are two statements
Statement I : Ligaments are dense irregular tissue.
Statement II : Cartilage is dense regular tissue.
In the light of the above statements, choose the correct answer from the options given below:
Statement I : Ligaments are dense irregular tissue.
Statement II : Cartilage is dense regular tissue.
In the light of the above statements, choose the correct answer from the options given below:
166.
Which of the following functions is carried out by
cytoskeleton in a cell ?
167.
Match List I with List II.
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
168.
Which of the following statements is correct ?
169.
Which one of the following symbols representsmating between relatives in human pedigree analysis?
170.
Once the undigested and unabsorbed substances
enter the caecum, their backflow is prevented by:
171.
Which one of the following techniques does not
serve the purpose of early diagnosis of a disease
for its early treatment ?
172.
Given below are two statements :
Statement I : Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Statement II : When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
In the light of the above statements, choose the correct answer from the options given below :
Statement I : Low temperature preserves the enzyme in a temporarily inactive state whereas high temperature destroys enzymatic activity because proteins are denatured by heat.
Statement II : When the inhibitor closely resembles the substrate in its molecular structure and inhibits the activity of the enzyme, it is known as competitive inhibitor.
In the light of the above statements, choose the correct answer from the options given below :
173.
Match List I with List II.
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
174.
Given below are two statements :
Statement I : Vas deferens receives a duct from seminal vesicle and opens into urethra as the ejaculatory duct.
Statement II : The cavity of the cervix is called cervical canal which along with vagina forms birth canal.
In the light of the above statements, choose the correct answer from the options given below :
Statement I : Vas deferens receives a duct from seminal vesicle and opens into urethra as the ejaculatory duct.
Statement II : The cavity of the cervix is called cervical canal which along with vagina forms birth canal.
In the light of the above statements, choose the correct answer from the options given below :
175.
Match List I with List II
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
176.
Vital capacity of lung is
177.
Select the correct group/set of Australian
Marsupials exhibiting adaptive radiation.
178.
Match List I with List II
Choose the correct answer from the options given below

Choose the correct answer from the options given below
179.
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R.
Assertion A: Amniocentesis for sex determination is one of the strategies of Reproductive and Child Health Care Programme.
Reason R: Ban on amniocentesis checks increasing menace of female foeticide.
In the light of the above statements, choose the correct answer from the options given below :
Assertion A: Amniocentesis for sex determination is one of the strategies of Reproductive and Child Health Care Programme.
Reason R: Ban on amniocentesis checks increasing menace of female foeticide.
In the light of the above statements, choose the correct answer from the options given below :
180.
Given below are two statements :
Statemnent I : RNA mutates at a faster rate.
Statement II : Viruses having RNA genome and shorter life span mutate and evolve faster.
In the light of the above statements, choose the correct answer from the options given below:
Statemnent I : RNA mutates at a faster rate.
Statement II : Viruses having RNA genome and shorter life span mutate and evolve faster.
In the light of the above statements, choose the correct answer from the options given below:
181.
Match List I with List II.
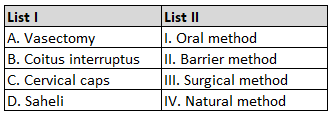
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
182.
Given below are two statements:
Statement I : Electrostatic precipitator is most widely used in thermal power plant.
Statemnent II : Electrostatic precipitator in thermal power plant removes ionising radiations.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Electrostatic precipitator is most widely used in thermal power plant.
Statemnent II : Electrostatic precipitator in thermal power plant removes ionising radiations.
In the light of the above statements, choose the most appropriate answer from the options given below :
183.
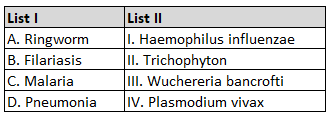
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
184.
Match List I with List II.
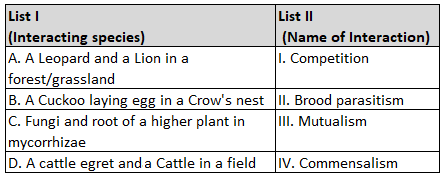
Choose the correct answer from the options given below:

Choose the correct answer from the options given below:
185.
Which of the following statements are correct?
A. Basophils are most abundant cells of the total WBCs
B. Basophils secrete histamine, serotonin and heparin
C. Basophils are involved in inflammatory response
D. Basophils have kidney shaped nucleus
E. Basophils are agranulocytes
Choose the correct answer from the options given below:
A. Basophils are most abundant cells of the total WBCs
B. Basophils secrete histamine, serotonin and heparin
C. Basophils are involved in inflammatory response
D. Basophils have kidney shaped nucleus
E. Basophils are agranulocytes
Choose the correct answer from the options given below:
186.
Match List I with List II.
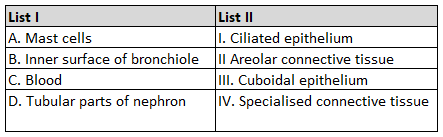
Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
187.
In cockroach, excretion is brought about by:
A. Phallic gland
B. Urecose gland
C. Nephrocytes
D. Fat body
E. Collaterial glands
Choose the correct answer from the options given below:
A. Phallic gland
B. Urecose gland
C. Nephrocytes
D. Fat body
E. Collaterial glands
Choose the correct answer from the options given below:
188.
Given below are two statements:
Statement I : During G0, phase of cell cycle, the cell is metabolically inactive.
Statement II : The centrosome undergoes duplication during S phase of interphase.
In the light of the above statements, choose the most appropriate answer from the options given below:
Statement I : During G0, phase of cell cycle, the cell is metabolically inactive.
Statement II : The centrosome undergoes duplication during S phase of interphase.
In the light of the above statements, choose the most appropriate answer from the options given below:
189.
Select the correct statements with reference to
chordates.
A. Presence of mid-dorsal, solid and double nerve cord.
B. Presence of closed circulatory system.
C. Presence of paired pharyngeal gillslits.
D. Presence of dorsal heart.
E. Triploblastic pseudocoelomate animals.
Choose the correct answer from the options given below:
A. Presence of mid-dorsal, solid and double nerve cord.
B. Presence of closed circulatory system.
C. Presence of paired pharyngeal gillslits.
D. Presence of dorsal heart.
E. Triploblastic pseudocoelomate animals.
Choose the correct answer from the options given below:
190.
Match List I with List II

Choose the correct answer from the options given below :

Choose the correct answer from the options given below :
191.
Which one of the following is the sequence on
corresponding coding strand, if the sequence on
mRNA formed is as follows:
5' AUCGAUCGAUCGAUCGAUCG AUCG AUCG 3'?
5' AUCGAUCGAUCGAUCGAUCG AUCG AUCG 3'?
192.
Which of the following is characteristic feature of
cockroach regarding sexual dimorphism?
193.
Which of the following statements are correct
regarding skeletal muscle?
A. Muscle bundles are held together by collagenous connective tissue layer called fascicle.
B. Sarcoplasmic reticulum of muscle fibre is a store house of calcium ions.
C. Striated appearance of skeletal muscle fibre is due to distribution pattern of actin and myosin proteins.
D. M line is considered as functional unit of contraction called sarcomere.
Choose the most appropriate answer from the options given below:
A. Muscle bundles are held together by collagenous connective tissue layer called fascicle.
B. Sarcoplasmic reticulum of muscle fibre is a store house of calcium ions.
C. Striated appearance of skeletal muscle fibre is due to distribution pattern of actin and myosin proteins.
D. M line is considered as functional unit of contraction called sarcomere.
Choose the most appropriate answer from the options given below:
194.
The unique mammalian characteristics are :
195.
Which one of the following is NOT an advantage
of inbreeding?
196.
The parts of human brain that helps in regulation
of sexual behaviour, expression of excitement,
pleasure, rage, fear etc. are:
197.
Which of the following statements are correct?
A. An excessive loss of body fluid from the body switches off osmoreceptors.
B. ADH facilitates water reabsorption to prevent diuresis.
C. ANF causes vasodilation.
D. ADH causes increase in blood pressure.
E. ADH is responsible for decrease in GFR.
Choose the correct answer from the options given below:
A. An excessive loss of body fluid from the body switches off osmoreceptors.
B. ADH facilitates water reabsorption to prevent diuresis.
C. ANF causes vasodilation.
D. ADH causes increase in blood pressure.
E. ADH is responsible for decrease in GFR.
Choose the correct answer from the options given below:
198.
Which of the following are NOT under the control
of thyroid hormone?
A. Maintenance of water and electrolyte balance.
B. Regulation of basal metabolic rate.
C. Normal rhythm of sleep-wake cycle.
D. Development of immune system.
E. Support the process of R.B.Cs formation.
Choose the correct answer from the options given below:
A. Maintenance of water and electrolyte balance.
B. Regulation of basal metabolic rate.
C. Normal rhythm of sleep-wake cycle.
D. Development of immune system.
E. Support the process of R.B.Cs formation.
Choose the correct answer from the options given below:
199.
The phenomenon of pleiotropism refers to
200.
In which blood corpuscles, the HIV undergoes
replication and produces progeny viruses ?