Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 20
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
Noble gases are named because of their inertness
towards reactivity. Identify an incorrect statement
about them.
(1). Noble gases are sparingly soluble in water
(2). Noble gases have very high melting and boiling points.
(3). Noble gases have weak dispersion forces.
(4). Noble gases have large positive values of electron gain enthalpy.
(1). Noble gases are sparingly soluble in water
(2). Noble gases have very high melting and boiling points.
(3). Noble gases have weak dispersion forces.
(4). Noble gases have large positive values of electron gain enthalpy.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Statement I : Acid strength increases in the order given as
HF << HCI << HBr << HI.
Statement II : As the size of the elements F, Cl, Br, I increases down the group, the bond strength of HF, HCl, HBr and HI decreases and so the acid strength increases.
In the light of the above statements, choose thecorrect answer from the options given below.
(1). Both Statement I and Statement II are true.
(2). Both Statement I and Statement II are false.
(3). Statement I is correct but Statement Il isfalse.
(4). Statement I is incorrect but Statement II istrue.
HF << HCI << HBr << HI.
Statement II : As the size of the elements F, Cl, Br, I increases down the group, the bond strength of HF, HCl, HBr and HI decreases and so the acid strength increases.
In the light of the above statements, choose thecorrect answer from the options given below.
(1). Both Statement I and Statement II are true.
(2). Both Statement I and Statement II are false.
(3). Statement I is correct but Statement Il isfalse.
(4). Statement I is incorrect but Statement II istrue.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match the Xenon compounds in Column-I with its structure in Column-II and assign the correct code
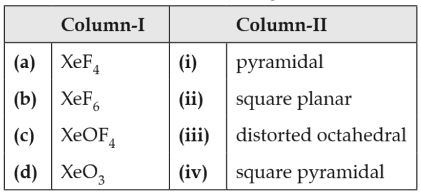
(1). (a) - (ii), (b) -(iii), (c) - (iv), (d) - (i)
(2). (a) - (ii), (b) - (iii), (c) - (i), (d) - (iv)
(3). (a) - (iii), (b) - (iv), (c) - (i), (d) - (ii)
(4). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)

(1). (a) - (ii), (b) -(iii), (c) - (iv), (d) - (i)
(2). (a) - (ii), (b) - (iii), (c) - (i), (d) - (iv)
(3). (a) - (iii), (b) - (iv), (c) - (i), (d) - (ii)
(4). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct order of atomic radii in group 13 elements is
(1). B < Ga < Al < In < Tl
(2). B < Ga < Al < Tl < In
(3). B < Al < Ga < In < Tl
(4). B < Al < In < Ga < Tl
(1). B < Ga < Al < In < Tl
(2). B < Ga < Al < Tl < In
(3). B < Al < Ga < In < Tl
(4). B < Al < In < Ga < Tl
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which is the correct statement for the given acids ?
(1). Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid
(2). Phosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid
(3). Both are triprotic acids
(4). Both are diprotic acids
(1). Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid
(2). Phosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid
(3). Both are triprotic acids
(4). Both are diprotic acids
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules ?
(1). Cl2 > Br2 > F2 > I2
(2). Br2 > I2 > F2 > Cl2
(3). F2 > Cl2 Br2 > I2
(4). I2 > Br2 > Cl2 > F2
(1). Cl2 > Br2 > F2 > I2
(2). Br2 > I2 > F2 > Cl2
(3). F2 > Cl2 Br2 > I2
(4). I2 > Br2 > Cl2 > F2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The following metal ion activates many enzymes,
participates in the oxidation of glucose to produce
ATP and with Na, is responsible for the transmission
of nerve signals.
(1). Copper
(2). Calcium
(3). Potassium
(4). Iron
(1). Copper
(2). Calcium
(3). Potassium
(4). Iron
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following oxoacid of sulphur has -O-O-linkage?
(1). H2SO4, sulphuric acid
(2). H2S2O8, peroxodisulphuric acid
(3). H2s2O7. pyrosulphuric acid
(4). H2SO3, sulphurous acid
(1). H2SO4, sulphuric acid
(2). H2S2O8, peroxodisulphuric acid
(3). H2s2O7. pyrosulphuric acid
(4). H2SO3, sulphurous acid
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
ldentify the correct statements from the following:
(a) CO2(g) is used as refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains twelve six carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert alcohols into gasoline.
(d) CO is colorless and odourless gas.
(1). (a) and (c) only
(2). (b) and (c) only
(3). (c) and (d) only
(4). (a), (b) and (c) only
(a) CO2(g) is used as refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains twelve six carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert alcohols into gasoline.
(d) CO is colorless and odourless gas.
(1). (a) and (c) only
(2). (b) and (c) only
(3). (c) and (d) only
(4). (a), (b) and (c) only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match the following :
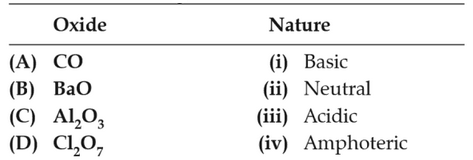
(1). (a)-(ii), (b)-(i), (c)-(iv), (d)-(iii)
(2). (a)-(iii), (b)-(iv), (c)-(i), (d)-(ii)
(3). (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(4). (a)-(i), (b)-(ii), (c)-(iii), (d)-(iv)

(1). (a)-(ii), (b)-(i), (c)-(iv), (d)-(iii)
(2). (a)-(iii), (b)-(iv), (c)-(i), (d)-(ii)
(3). (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(4). (a)-(i), (b)-(ii), (c)-(iii), (d)-(iv)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
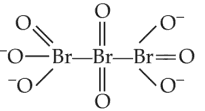
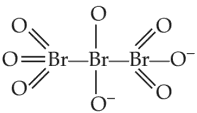
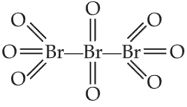
The correct structure or tribromo octaoxide is
(1).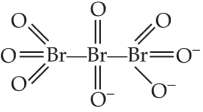
(2).
(3).
(4).
(1).
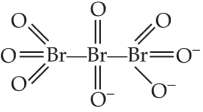
(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which is the correct thermal stability order for H2E
(E = O, S, Se, Te and Po)?
(1). H2O < H2S < H2Se < H2Te < H2Po
(2). H2Po < H2Te < H2Se < H2S < H2O
(3). H2Se < H2Te < H2Po < H2O < H2S
(4). H2S < H2O < H2Se < H2Te < H2Po
(E = O, S, Se, Te and Po)?
(1). H2O < H2S < H2Se < H2Te < H2Po
(2). H2Po < H2Te < H2Se < H2S < H2O
(3). H2Se < H2Te < H2Po < H2O < H2S
(4). H2S < H2O < H2Se < H2Te < H2Po
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following is incorrect statement ?
(1). SiCl4 is easily hydrolysed.
(2). GeX4 (X = F, Cl, Br, I) is more stable than GeX2.
(3). SnF4 is ionic in nature.
(4). PbF4 is covalent in nature.
(1). SiCl4 is easily hydrolysed.
(2). GeX4 (X = F, Cl, Br, I) is more stable than GeX2.
(3). SnF4 is ionic in nature.
(4). PbF4 is covalent in nature.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following species is not stable?
(1). [GeCl6]2–
(2). [Sn(OH)6]2–
(3). [SiCl6]2–
(4). [SiF6]2–
(1). [GeCl6]2–
(2). [Sn(OH)6]2–
(3). [SiCl6]2–
(4). [SiF6]2–
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following oxides is most acidic in nature ?
(1). CaO
(2). BaO
(3). BeO
(4). MgO
(1). CaO
(2). BaO
(3). BeO
(4). MgO
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following statements is not true for halogens ?
(1). Chlorine has the highest electron-gain enthalpy.
(2). All halogens except fluorine show positive oxidation states. .
(3). All are oxidizing agents.
(4). All form monobasic oxyacids
(1). Chlorine has the highest electron-gain enthalpy.
(2). All halogens except fluorine show positive oxidation states. .
(3). All are oxidizing agents.
(4). All form monobasic oxyacids
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the following elements is unable to form \(\text{MF}_^{3-} ion ?
(1). In
(2). B
(3). Al
(4). Ga
(1). In
(2). B
(3). Al
(4). Ga
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
When copper is heated with conc. HNO3 it produces
(1). Cu(NO3)2 and NO
(2). Cu(NO3)2, NO and NO2
(3). Cu(NO3)2 and N2O
(4). Cu(NO3)2 and NO2
(1). Cu(NO3)2 and NO
(2). Cu(NO3)2, NO and NO2
(3). Cu(NO3)2 and N2O
(4). Cu(NO3)2 and NO2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
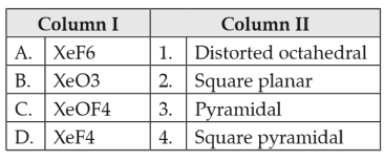
Match the compounds given in column I with the hybridisation and shape given in column II and mark the correct option.
(1). A - 1, B - 2, C - 4, D - 3
(2). A - 4, B - 3, C - 1, D - 2
(3). A - 4, B - 1, C - 2, D - 3
(4). A - 1, B - 3, C - 4, D - 2

(1). A - 1, B - 2, C - 4, D - 3
(2). A - 4, B - 3, C - 1, D - 2
(3). A - 4, B - 1, C - 2, D - 3
(4). A - 1, B - 3, C - 4, D - 2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the following statements is correct when SO2 is passed through acidified K2Cr2O7 solution ?
(1). The solution is decolourized
(2). SO2 is reduced
(3). Green Cr2(SO4)3 is formed
(4). The solution turns blue
(1). The solution is decolourized
(2). SO2 is reduced
(3). Green Cr2(SO4)3 is formed
(4). The solution turns blue
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
