Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 9
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
The average thermal energy for a monoatomic gas is (where,\(k_ B\) is Boltzmann constant and T is absolute temperature.)
(1). \(\displaystyle \frac{3}{2}k_B\,T\)
(2). \(\displaystyle \frac{5}{2}k_B\,T\)
(3). \(\displaystyle \frac{7}{2}k_B\,T\)
(4). \(\displaystyle \frac{1}{2}k_B\,T\)
(1). \(\displaystyle \frac{3}{2}k_B\,T\)
(2). \(\displaystyle \frac{5}{2}k_B\,T\)
(3). \(\displaystyle \frac{7}{2}k_B\,T\)
(4). \(\displaystyle \frac{1}{2}k_B\,T\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The following graph represents the T - V curves of an ideal gas ( where T is the temperature and V the volume ) at three pressures P1 , P2 and P3 compared with those of Charles's law represented as dotted lines .
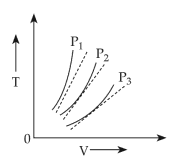
(1). P2 > P1 > P3
(2). P1 > P2 > P3
(3). P3 > P2 > P1
(4). P1 > P3 > P2

(1). P2 > P1 > P3
(2). P1 > P2 > P3
(3). P3 > P2 > P1
(4). P1 > P3 > P2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match Column l with Column ll andchoose the correct match fromthegiven choices.
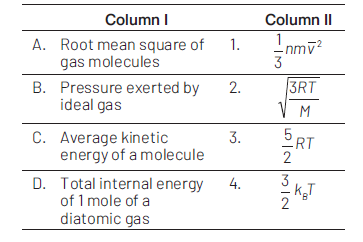
(1). A-3, B-1, C-4, D-2
(2). A-2, B-3, C-4, D-1
(3). A-2, B-1, C-4, D-3
(4). A-3, B-2, C-1, D-4

(1). A-3, B-1, C-4, D-2
(2). A-2, B-3, C-4, D-1
(3). A-2, B-1, C-4, D-3
(4). A-3, B-2, C-1, D-4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The mean free path l for a gas,with molecular diameter d and number density n can be expressed as
(1). \(\displaystyle \frac{1}{\sqrt{2}n\pi d^2}\)
(2). \(\displaystyle \frac{1}{\sqrt{2}n^2\pi d^2}\)
(3). \(\displaystyle \frac{1}{\sqrt{2}n^2\pi^2 d^2}\)
(4). \(\displaystyle \frac{1}{\sqrt{2}n\pi d}\)
(1). \(\displaystyle \frac{1}{\sqrt{2}n\pi d^2}\)
(2). \(\displaystyle \frac{1}{\sqrt{2}n^2\pi d^2}\)
(3). \(\displaystyle \frac{1}{\sqrt{2}n^2\pi^2 d^2}\)
(4). \(\displaystyle \frac{1}{\sqrt{2}n\pi d}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A cylinder contains hydrogen gas at pressure of 249 kPa and temperature 27°C. Its density is (\(R = 8.3\, J mol^{ −1} K^{−1}\) )
(1). \(0.2\, kg/m^3\)
(2). \( 0.1\, kg/m^3\)
(3). \( 0.02 \, kg/m^3\)
(4). \(0.5 \, kg/m^3\)
(1). \(0.2\, kg/m^3\)
(2). \( 0.1\, kg/m^3\)
(3). \( 0.02 \, kg/m^3\)
(4). \(0.5 \, kg/m^3\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Increase in temperature of a gas filled in a container would lead to
(1). increase in its kinetic energy
(2). decrease in its pressure
(3). decrease in intermolecular distance
(4). increase in its mass
(1). increase in its kinetic energy
(2). decrease in its pressure
(3). decrease in intermolecular distance
(4). increase in its mass
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
At what temperature will the rms speed of oxygen molecules become just sufficient for escaping from the Earth’s atmosphere?
(Given: mass of oxygen molecule,\(m = 2.76 × 10^−{26}\, kg\) ,Boltzmann’s constant\(k_B = 1.38 10^{-23} J K^{ −1}\))
(1). \(5.016 \times 10^4\, K\)
(2). \(8.326 \times 10^4\, K\)
(3). \(2.508 \times 10^4\, K\)
(4). \(1.254 \times 10^4\, K\)
(Given: mass of oxygen molecule,\(m = 2.76 × 10^−{26}\, kg\) ,Boltzmann’s constant\(k_B = 1.38 10^{-23} J K^{ −1}\))
(1). \(5.016 \times 10^4\, K\)
(2). \(8.326 \times 10^4\, K\)
(3). \(2.508 \times 10^4\, K\)
(4). \(1.254 \times 10^4\, K\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A gas mixture consists of 2 moles of \(O_2\) and 4 moles of \(A_r\) at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
(1). 4 RT
(2). 15 RT
(3). 9 RT
(4). 11 RT
(1). 4 RT
(2). 15 RT
(3). 9 RT
(4). 11 RT
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The molecules of a given mass of a gas have r.m.s. velocity of \(200\, \text{ms}^{−1}\) at \(27^\circ \text{C}\) and \(1.0 \times 10^5 \text{Nm}^{−2}\) pressure. When the temperature and pressure of the gas are respectively, \(127^ \circ \text{C}\) and \(0.05 \times 10^5 \text{Nm}^{ −2}\) , the rms velocity of its molecules in \(\text{ms}^{ −1}\) is
(1). \(\displaystyle \frac{400}{\sqrt{3}}\)
(2). \(\displaystyle \frac{100\sqrt{2}}{3}\)
(3). \(\displaystyle \frac{100}{3}\)
(4). \(\displaystyle100\sqrt{2}\)
(1). \(\displaystyle \frac{400}{\sqrt{3}}\)
(2). \(\displaystyle \frac{100\sqrt{2}}{3}\)
(3). \(\displaystyle \frac{100}{3}\)
(4). \(\displaystyle100\sqrt{2}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
