Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 19
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
A Carnot engine has an efficiency of 50% when its source is at a temperature \(327^\circ C\). The temperature of the sink is
(1). \(15^\circ C\)
(2). \(100^\circ C\)
(3). \(200^\circ C\)
(4). \(27^\circ C\)
(1). \(15^\circ C\)
(2). \(100^\circ C\)
(3). \(200^\circ C\)
(4). \(27^\circ C\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The volume occupied by the molecules contained in 4.5 kg water at STP, if the intermolecular forces vanish away is
(1). \(5.6 \times 10^6\, m^3\)
(2). \(5.6 \times 10^3\, m^3\)
(3). \(5.6 \times 10^{-3}\, m^3\)
(4). \(5.6\,m^3\)
(1). \(5.6 \times 10^6\, m^3\)
(2). \(5.6 \times 10^3\, m^3\)
(3). \(5.6 \times 10^{-3}\, m^3\)
(4). \(5.6\,m^3\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Two gases A and B are filled at the same pressure in separate cylinders with movable pistons of radius \(\text{r}_\text{A}\) and \(\text{r}_\text{B}\), respectively. On supplyig an equal amount of heat to both the systems reversibly under constant presurre, the pistons of gas A nd B are displaced by 16 cm and 9 cm, respectively. If the change in their internal energy is the same, then the ration \(\displaystyle \frac{\text{r}_\text{A}}{\text{r}_\text{B}}\) is equal to
(1). \(\displaystyle \frac{\sqrt{3}}{2}\)
(2). \(\displaystyle \frac{4}{3}\)
(3). \(\displaystyle \frac{3}{4}\)
(4). \(\displaystyle \frac{2}{\sqrt{3}}\)
(1). \(\displaystyle \frac{\sqrt{3}}{2}\)
(2). \(\displaystyle \frac{4}{3}\)
(3). \(\displaystyle \frac{3}{4}\)
(4). \(\displaystyle \frac{2}{\sqrt{3}}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A container has two chambers of volumes \(\text{V}_1\,=\, 2\,\text{litres}\) and \(\text{V}_2\,=\, 3\,\text{litres}\) separated by a partition made of a thermal insulator. The chambers contain n1 = 5 and n2 = 4 moles of ideal gas at pressure p1 = 1 and p2 = 2 atm, respectively. When the partition is removed, the mixture attains equilibrium pressure of
(1). 1.8 atm
(2). 1.3 atm
(3). 1.6 atm
(4). 1.4 atm
(1). 1.8 atm
(2). 1.3 atm
(3). 1.6 atm
(4). 1.4 atm
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressures at temperature \(27^\circ\ \text{C}\). The mass of oxygen withdrawn from the cylinder is nearly equal to:
[Given, \(\text{R}\,=\, \frac{100}{12}\text{J mol}^{-1}\text{K}^{-1}\) and molecular mass of \(\text{O}_2\,=\, 32\), 1 atm pressure \(=\,1.01 \times 10^5 \text{N/m}\)]
(1). 0.156 kg
(2). 0.125 kg
(3). 0.144 kg
(4). 0.116 kg
[Given, \(\text{R}\,=\, \frac{100}{12}\text{J mol}^{-1}\text{K}^{-1}\) and molecular mass of \(\text{O}_2\,=\, 32\), 1 atm pressure \(=\,1.01 \times 10^5 \text{N/m}\)]
(1). 0.156 kg
(2). 0.125 kg
(3). 0.144 kg
(4). 0.116 kg
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A thermodynamic system is taken through the cycle abcda. The work done by the gas along the path bc is: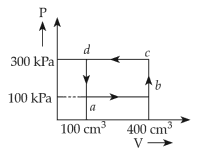
(1). -90 J
(2). -60 J
(3). zero
(4). 30 J

(1). -90 J
(2). -60 J
(3). zero
(4). 30 J
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The temperature of a gas is \(−50^\circ C\). To what temperature the gas should be heated so that the rms speed is increased by 3 times?
(1). \(3295^\circ C\)
(2). \(3097\,K\)
(3). \(223\,K\)
(4). \(669^\circ C\)
(1). \(3295^\circ C\)
(2). \(3097\,K\)
(3). \(223\,K\)
(4). \(669^\circ C\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
An ideal gas undergoes four different processes from the same initial state as shown in the figure below. Those processes are adiabatic, isothermal, isobaric and isochoric. The curve which represents the adiabatic process among 1, 2, 3 and 4 is
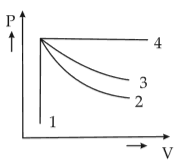
(1). 1
(2). 2
(3). 3
(4). 4

(1). 1
(2). 2
(3). 3
(4). 4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A cup of coffee cools from \(90^\circ C\) to \(80^\circ C\) in t minutes, when the room temperature is \(20^\circ C\). The time taken by a similar cup of coffee to cool from \(80^\circ C\) to \(60^\circ C\) at a room temperature same at \(20^\circ C\) is
(1). \(\displaystyle \frac{5}{13}\, t\)
(2). \(\displaystyle \frac{13}{10}\, t\)
(3). \(\displaystyle \frac{13}{5}\, t\)
(4). \(\displaystyle \frac{10}{13}\, t\)
(1). \(\displaystyle \frac{5}{13}\, t\)
(2). \(\displaystyle \frac{13}{10}\, t\)
(3). \(\displaystyle \frac{13}{5}\, t\)
(4). \(\displaystyle \frac{10}{13}\, t\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Two cylinders A and B of equal capacity are connected to each other via a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stop cock is suddenly opened. The process is
(1). adiabatic
(2). isochoric
(3). isobaric
(4). isothermal
(1). adiabatic
(2). isochoric
(3). isobaric
(4). isothermal
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The quantities of heat required to raise the temperarture of two solid copper spheres of radii \(r_1\) and \(r_2\) (\(r_1\,=\,1.5\,r_2\)) through 1 K are in the ratio
(1). 9/5
(2). 3/2
(3). 5/3
(4). 27/8
(1). 9/5
(2). 3/2
(3). 5/3
(4). 27/8
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which of the following processes, heat is neither absorbed nor released by a system?
(1). Adiabatic
(2). Isobaric
(3). Isochoric
(4). Isothermal
(1). Adiabatic
(2). Isobaric
(3). Isochoric
(4). Isothermal
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A sample of 0.1 g of water at 100°C and normal pressure (\(1.013 \times 10^5 Nm^{-2}\) ) requires 54 cal of heat energy to convert to steam at 100°C. If the volume of the steam produced is 167.1 cc, the change in internal energy of the sample,
(1). 42.2 J
(2). 208.7 J
(3). 104.3 J
(4). 84.5 J
(1). 42.2 J
(2). 208.7 J
(3). 104.3 J
(4). 84.5 J
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The volume (V) of a monoatomic gas varies with its temperature (T), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state A to state B, is
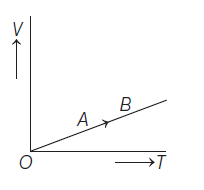
(1). \(\displaystyle \frac{1}{3}\)
(2). \(\displaystyle \frac{2}{3}\)
(3). \(\displaystyle \frac{2}{5}\)
(4). \(\displaystyle \frac{2}{7}\)

(1). \(\displaystyle \frac{1}{3}\)
(2). \(\displaystyle \frac{2}{3}\)
(3). \(\displaystyle \frac{2}{5}\)
(4). \(\displaystyle \frac{2}{7}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The efficiency of an ideal heat engine working between the freezing point and boiling point of water, is
(1). 12.5%
(2). 6.25%
(3). 20%
(4). 26.80%
(1). 12.5%
(2). 6.25%
(3). 20%
(4). 26.80%
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Thermodynamic processes are indicated in the following diagram
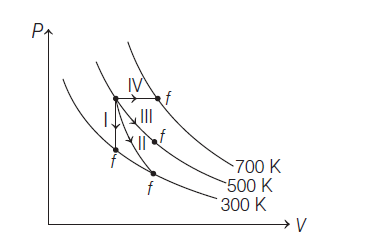
Match the following:
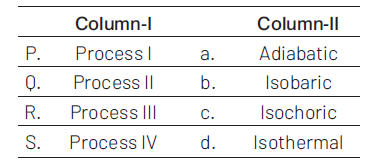
(1). \(P\rightarrow a\), \(Q\rightarrow c\), \(R\rightarrow d\), \(S\rightarrow b\)
(2). \(P\rightarrow c\), \(Q\rightarrow a\), \(R\rightarrow d\), \(S\rightarrow b\)
(3). \(P\rightarrow c\), \(Q\rightarrow d\), \(R\rightarrow b\), \(S\rightarrow a\)
(4). \(P\rightarrow d\), \(Q\rightarrow b\), \(R\rightarrow a\), \(S\rightarrow c\)

Match the following:

(1). \(P\rightarrow a\), \(Q\rightarrow c\), \(R\rightarrow d\), \(S\rightarrow b\)
(2). \(P\rightarrow c\), \(Q\rightarrow a\), \(R\rightarrow d\), \(S\rightarrow b\)
(3). \(P\rightarrow c\), \(Q\rightarrow d\), \(R\rightarrow b\), \(S\rightarrow a\)
(4). \(P\rightarrow d\), \(Q\rightarrow b\), \(R\rightarrow a\), \(S\rightarrow c\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A Carnot engine having an efficiency of 110 as heat engine, is used as a refrigerator. If the workdone on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
(1). 1 J
(2). 90 J
(3). 99 J
(4). 100 J
(1). 1 J
(2). 90 J
(3). 99 J
(4). 100 J
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half.
(1). compressing the gas through adiabatic process will require more work to be done.
(2). compressing the gas isothermally or adiabatically will require the same amount of work.
(3). which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
(4). compressing the gas isothermally will require more work to be done.
(1). compressing the gas through adiabatic process will require more work to be done.
(2). compressing the gas isothermally or adiabatically will require the same amount of work.
(3). which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
(4). compressing the gas isothermally will require more work to be done.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant.The power required is (Take, 1 cal =4.2Joules)
(1). 23.65 W
(2). 236.5 W
(3). 2365 W
(4). 2.365 W
(1). 23.65 W
(2). 236.5 W
(3). 2365 W
(4). 2.365 W
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
