Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 18
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
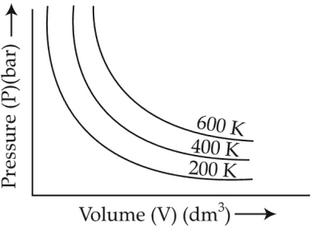
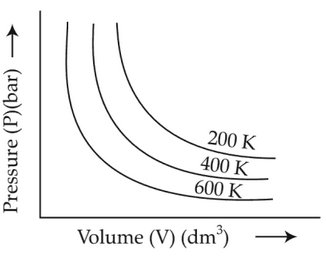
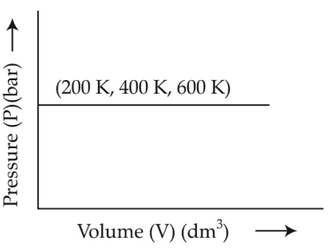
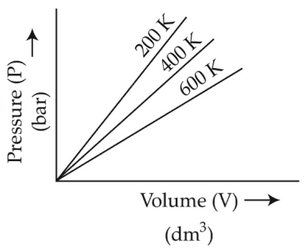
Choose the correct option for graphical
representation of Boyle's law, which shows a graph
of pressure vs. volume of a gas at different
temperatures:
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The standard heat of formation, in kcal/mol of \(Ba^{2+}\) is:
[Given : standard heat of formation of \(\text{So}^{2-}_4\) ion (aq) = - 216 kcal/mol, standard heat of crystallisation of \(\text{BaSO}_4(s)\) = - 4.5 kcal/mol, standard heat of formation of \(\text{ BaSO}_4(s)\) = - 349 kcal/mol]
(1). + 220.5
(2). - 128.5
(3). - 133.0
(4). + 133.0
(1). + 220.5
(2). - 128.5
(3). - 133.0
(4). + 133.0
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
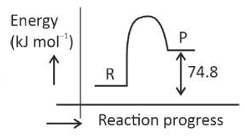
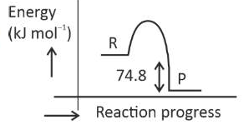
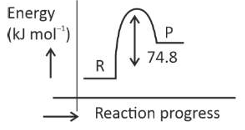
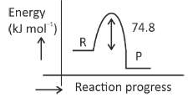
\(\text{C(s) + 2H}_2 \left( g \right) \rightarrow CH_4(g)\);\(\Delta H = -74.8 kJ mol^{-l}\)
Which of the following diagrams gives an accurate representation of the above reaction?
[R → reactants; P → products]
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List-I with List-II
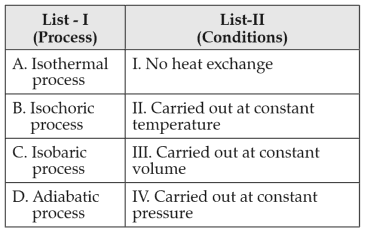
Choose the correct answer from the options given below:
(1). A-I, B=II, C-III, D-IV
(2). A-II, B-III, C-IV. D-I
(3). A-IV, B-III, C-II, D-I
(4). A-IV, B-II, C-III, D-I

Choose the correct answer from the options given below:
(1). A-I, B=II, C-III, D-IV
(2). A-II, B-III, C-IV. D-I
(3). A-IV, B-III, C-II, D-I
(4). A-IV, B-II, C-III, D-I
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which of the following processes entropy form increases ?
(1). A liquid evaporates to vapour
(2). Temperature of a crystalline solid lowered from 130 K to 0 K
(3). \(2\text{NaHCO}_3\left(g\right)\,\rightarrow\,\text{Na}_2\text{CO}_3\left(s\right)\) +\(\text{CO}_2\left(g\right)\,+\,\text{H}_2\text{O}\left(g\right)\)
(4). \( \text{Cl}_2(g) \rightarrow 2\text{Cl}(g) \)
(1). A liquid evaporates to vapour
(2). Temperature of a crystalline solid lowered from 130 K to 0 K
(3). \(2\text{NaHCO}_3\left(g\right)\,\rightarrow\,\text{Na}_2\text{CO}_3\left(s\right)\) +\(\text{CO}_2\left(g\right)\,+\,\text{H}_2\text{O}\left(g\right)\)
(4). \( \text{Cl}_2(g) \rightarrow 2\text{Cl}(g) \)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The work done during reversible isothermal expansion of one mole of hydrogen gas at \(25^\circ \text{C}\) from pressure of \(20\) atmosphere to \(10\) atmosphere is:
(Given \(\text{R}\,=\,2.0\,cal K^{-1}mol^{-1}\))
(1). 413.14 calories
(2). 100 calories
(3). 0 calorie
(4). -413.14 calories
(Given \(\text{R}\,=\,2.0\,cal K^{-1}mol^{-1}\))
(1). 413.14 calories
(2). 100 calories
(3). 0 calorie
(4). -413.14 calories
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements : one is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : In equation \(\delta_r\, G\, =\, -nFE_{\text{cell}}\), value of \(\delta_r\, G\) depends on n.
Reason R: \(E_{\text{cell}}\) is an intensive property and \(\delta_r\, G\) is an extensive property.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Assertion A : In equation \(\delta_r\, G\, =\, -nFE_{\text{cell}}\), value of \(\delta_r\, G\) depends on n.
Reason R: \(E_{\text{cell}}\) is an intensive property and \(\delta_r\, G\) is an extensive property.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which amongst the following options is the correct
relation between change in enthalpy and change
in internal energy?
(1). \(\Delta H\,=\,\Delta U\, +\, \Delta n_g\, RT
(2). \(\Delta H\,-\,\Delta U\, =\,-\, \Delta n\, RT
(3). \(\Delta H\,+\,\Delta U\, =\, \Delta n\, RT
(4). \(\Delta H\,=\,\Delta U\, -\, \Delta n_g\, RT
(1). \(\Delta H\,=\,\Delta U\, +\, \Delta n_g\, RT
(2). \(\Delta H\,-\,\Delta U\, =\,-\, \Delta n\, RT
(3). \(\Delta H\,+\,\Delta U\, =\, \Delta n\, RT
(4). \(\Delta H\,=\,\Delta U\, -\, \Delta n_g\, RT
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
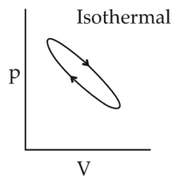
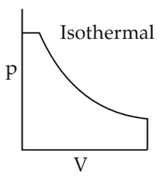
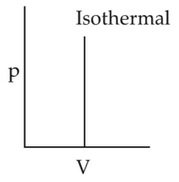
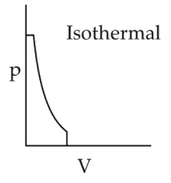
Which of the following p-V curve representsmaximum work done?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one among the following is the correct option for right relationship between CP and CV for one mole of ideal gas ?
(1). CP + CV= R
(2). CP-CV=R
(3). CP= RCV
(4). CV = RCP
(1). CP + CV= R
(2). CP-CV=R
(3). CP= RCV
(4). CV = RCP
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For irreversible expansion of an ideal gas under isothermal condition, the correct option is
(1).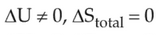
(2).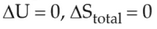
(3).
(4).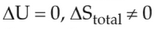
(1).
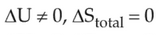
(2).
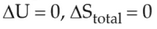
(3).

(4).
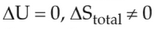
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For the reaction, 2Cl(g)→ C2(g), the correct option is:
(1). \(\Delta_r\, H \,>\, 0 \text{ and } \Delta_r \,S\, <\, 0\)
(2). \(\Delta_r\, H\, <\, 0 \text{ and } \Delta_r\, S \,>\, 0\)
(3). \(\Delta_r\, H\, <\, 0 \text{ and } \Delta_r\, S\, <\, 0\)
(4). \(\Delta_r\, H\, >\, 0 \text{ and } \Delta_r\, S\, >\, 0\)
(1). \(\Delta_r\, H \,>\, 0 \text{ and } \Delta_r \,S\, <\, 0\)
(2). \(\Delta_r\, H\, <\, 0 \text{ and } \Delta_r\, S \,>\, 0\)
(3). \(\Delta_r\, H\, <\, 0 \text{ and } \Delta_r\, S\, <\, 0\)
(4). \(\Delta_r\, H\, >\, 0 \text{ and } \Delta_r\, S\, >\, 0\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct option for free expansion of an ideal
gas under adiabatic condition is:
(1). \(q\, =\, 0,\, \Delta T\, <\,0\, \text{ and}\, w\,>\, 0\)
(2). \(q\, <\, 0,\, \Delta T\, =\,0\, \text{ and}\, w\,=\, 0\)
(3). \(q\, >\, 0,\, \Delta T\, >\,0\, \text{ and}\, w\,>\, 0\)
(4). \(q\, =\, 0,\, \Delta T\, =\,0\, \text{ and}\, w\,=\, 0\)
(1). \(q\, =\, 0,\, \Delta T\, <\,0\, \text{ and}\, w\,>\, 0\)
(2). \(q\, <\, 0,\, \Delta T\, =\,0\, \text{ and}\, w\,=\, 0\)
(3). \(q\, >\, 0,\, \Delta T\, >\,0\, \text{ and}\, w\,>\, 0\)
(4). \(q\, =\, 0,\, \Delta T\, =\,0\, \text{ and}\, w\,=\, 0\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Under isothermal condition, a gas at 300 K expands from 0.1 L to 0.25 L against a constant external pressure of 2 bar. The work done by the gas is
(1). 5 kJ
(2). 25 J
(3). 30 J
(4). -30 J
(1). 5 kJ
(2). 25 J
(3). 30 J
(4). -30 J
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which case change in entropy is negative ?
(1). Expansion of a gas at constant temperature
(2). Sublimation of solid to gas
(3). 2H(g) \(\rightarrow\) H2(g) (c)
(4). Evaporation of water
(1). Expansion of a gas at constant temperature
(2). Sublimation of solid to gas
(3). 2H(g) \(\rightarrow\) H2(g) (c)
(4). Evaporation of water
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A gas is allowed to expand in a well insulated container against a constant external pressure of 2.5 atm from an initial volume of 2.50 L to a final volume of 4.50 L. The change in internal energy \(\Delta \text{U}\) of the gas in joules will be
(1). +505 J
(2). 1136.25 J
(3). –500 J
(4). –505 J
(1). +505 J
(2). 1136.25 J
(3). –500 J
(4). –505 J
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct thermodynamic conditions for the spontaneous reaction at all temperatures is
(1). AH > 0 and AS < 0
(2). AH < 0 and AS > 0
(3). AH < 0 and AS < 0
(4). AH < 0 and AS = 0
(1). AH > 0 and AS < 0
(2). AH < 0 and AS > 0
(3). AH < 0 and AS < 0
(4). AH < 0 and AS = 0
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the following liquid - vapour equilibrium
Liquid \(\rightleftharpoons\) Vapour
Which of the following relations is correct ?
(1). \(\displaystyle \frac{\text{dlnP}}{\text{dT}}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}}\)
(2). \(\displaystyle \frac{\text{dlnP}}{\text{dT}^2}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{T}^2}\)
(3). \(\displaystyle \frac{\text{dlnP}}{\text{dT}}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}^2}\)
(4). \(\displaystyle \frac{\text{dlnG}}{\text{dT}^2}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}^2}\)
Liquid \(\rightleftharpoons\) Vapour
Which of the following relations is correct ?
(1). \(\displaystyle \frac{\text{dlnP}}{\text{dT}}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}}\)
(2). \(\displaystyle \frac{\text{dlnP}}{\text{dT}^2}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{T}^2}\)
(3). \(\displaystyle \frac{\text{dlnP}}{\text{dT}}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}^2}\)
(4). \(\displaystyle \frac{\text{dlnG}}{\text{dT}^2}\) = \(\displaystyle \frac{- \Delta text{H}_v}{\text{RT}^2}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
