Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 12
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
The correct order of decreasing basic strength of the given amines is :
(1). benzenamine > ethanamine > N-methylaniline > N-ethylethanamine
(2). N-methylaniline > benzenamine > ethanamine > N-ethylethanamine
(3). N-ethylethanamine > ethanamine > benzenamine > N-methylaniline
(4). N-ethylethanamine > ethanamine > N-methylaniline > benzenamine
(1). benzenamine > ethanamine > N-methylaniline > N-ethylethanamine
(2). N-methylaniline > benzenamine > ethanamine > N-ethylethanamine
(3). N-ethylethanamine > ethanamine > benzenamine > N-methylaniline
(4). N-ethylethanamine > ethanamine > N-methylaniline > benzenamine
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements :
Statement I : Benzenediazonium salt is prepared by the reaction of aniline with nitrous acid at 273 - 278 K. It decomposes easily in the dry state.
Statement Il : Insertion of iodine into the benzene ring is difficult and hence iodobenzene is prepared through the reaction of benzenediazonium salt with KI.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1). Statement I is incorrect but Statement II is correct
(2). Both Statement I and Statement II are correct
(3). Both Statement I and Statement Il are incorrect
(4). Statement I is correct but Statement Il is incorrect
Statement I : Benzenediazonium salt is prepared by the reaction of aniline with nitrous acid at 273 - 278 K. It decomposes easily in the dry state.
Statement Il : Insertion of iodine into the benzene ring is difficult and hence iodobenzene is prepared through the reaction of benzenediazonium salt with KI.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1). Statement I is incorrect but Statement II is correct
(2). Both Statement I and Statement II are correct
(3). Both Statement I and Statement Il are incorrect
(4). Statement I is correct but Statement Il is incorrect
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements:
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
(1). Statement I is correct, but Statement II is false
(2). Statement I is incorrect, but Statement II is true.
(3). Both Statement I and Statement I are true.
(4). Both Statement I and Statement II are false.
Statement I: Aniline does not undergo Friedel-Crafts alkylation reaction.
Statement II: Aniline cannot be prepared through Gabriel synthesis.
In the light of the above statements, choose the correct answer from the options given below:
(1). Statement I is correct, but Statement II is false
(2). Statement I is incorrect, but Statement II is true.
(3). Both Statement I and Statement I are true.
(4). Both Statement I and Statement II are false.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Identify the major product C formed in the following reaction sequence:

(1). \(butanamide\)
(2). \(\alpha-\text{bromobutanoic acid}\)
(3). \(propylamine\)
(4). \(butylamine\)

(1). \(butanamide\)
(2). \(\alpha-\text{bromobutanoic acid}\)
(3). \(propylamine\)
(4). \(butylamine\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following reactions will NOT give
primary amine as the product?
(1).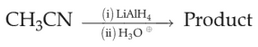
(2).
(3).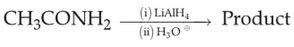
(4).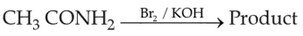
(1).
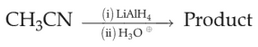
(2).

(3).
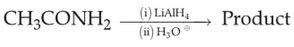
(4).
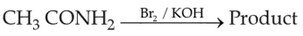
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements :
Statement I : Primary aliphatic amines react with HNO2 to give unstable diazonium salts.
Statement Il : Primary aromatic amines reactwith HNO2 to form diazonium salts which are stable even above 300 K.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but Statement II is correct.
Statement I : Primary aliphatic amines react with HNO2 to give unstable diazonium salts.
Statement Il : Primary aromatic amines reactwith HNO2 to form diazonium salts which are stable even above 300 K.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but Statement II is correct.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following amine will give the
carbylamine test ?
(1).
(2).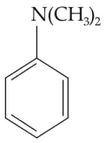
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct order of the basic strength of methyl substituted amines in aqueous solution is
(1). (CH3)3N > CH3NH2 > (CH3)2NH
(2). (CH3)3N > (CH3)2NH > CH3NH2
(3). CH3NH2 > (CH3) 2NH > (CH3)3N
(4). (CH3)2NH > CH3NH2 > (CH3)3N
(1). (CH3)3N > CH3NH2 > (CH3)2NH
(2). (CH3)3N > (CH3)2NH > CH3NH2
(3). CH3NH2 > (CH3) 2NH > (CH3)3N
(4). (CH3)2NH > CH3NH2 > (CH3)3N
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Nitration of aniline in strong acidic medium also gives m-nitroaniline because
(1). in acidic (strong) medium aniline is present as anilinium ion.
(2). in absence of substituents nitro group always goes to m-position.
(3). in electrophilic substitution reactions amino group is meta directive.
(4). inspite of substituents nitro group always goes to only m-position.
(1). in acidic (strong) medium aniline is present as anilinium ion.
(2). in absence of substituents nitro group always goes to m-position.
(3). in electrophilic substitution reactions amino group is meta directive.
(4). inspite of substituents nitro group always goes to only m-position.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following reactions is appropriate for converting acetamide to methanamine ?
(1). Gabriels phthalimide synthesis
(2). Carbylamine reaction
(3). Hoffmann hypobromamide reaction
(4). Stephens reaction
(1). Gabriels phthalimide synthesis
(2). Carbylamine reaction
(3). Hoffmann hypobromamide reaction
(4). Stephens reaction
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct increasing order of basic strength for the following compounds is
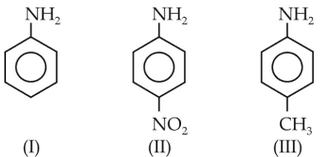
(1). II < I < III
(2). II < III < I
(3). III < I < II
(4). III < II < I

(1). II < I < III
(2). II < III < I
(3). III < I < II
(4). III < II < I
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct statement regarding the basicity of arylamines is
(1). Arylamines are generally more basic than alkylamines because the nitrogen lone - pair electrons are not delocalized by interaction with the aromatic ring л - electron system.
(2). Arylamines are generally more basic than alkylamines because of aryl group.
(3). Arylamines are generally more basic than alkylamines , because the nitrogen atom in arylamines is sp - hybridized.
(4). Arylamines are generally less basic than alkylamines because the nitrogen lone - pair electrons are delocalized by interaction with the aromatic ring л - electron system.
(1). Arylamines are generally more basic than alkylamines because the nitrogen lone - pair electrons are not delocalized by interaction with the aromatic ring л - electron system.
(2). Arylamines are generally more basic than alkylamines because of aryl group.
(3). Arylamines are generally more basic than alkylamines , because the nitrogen atom in arylamines is sp - hybridized.
(4). Arylamines are generally less basic than alkylamines because the nitrogen lone - pair electrons are delocalized by interaction with the aromatic ring л - electron system.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
