Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 12
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
Which of the following alkane cannot be made in good yield by Wurtz reaction?
(1). 2,3-Dimethylbutane
(2). n-Heptane
(3). n-Butane
(4). n-Hexane
(1). 2,3-Dimethylbutane
(2). n-Heptane
(3). n-Butane
(4). n-Hexane
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) :
Reason (R) : lodine is a better leaving group because of its large size.
In the light of the above statements, choose the correct answer from the options given below :
(1). A is false but R is true
(2). Both A and R are true and R is the correct explanation of A
(3). Both A and R are true but R is not the correct explanation of A
(4). A is true but R is false
Assertion (A) :

Reason (R) : lodine is a better leaving group because of its large size.
In the light of the above statements, choose the correct answer from the options given below :
(1). A is false but R is true
(2). Both A and R are true and R is the correct explanation of A
(3). Both A and R are true but R is not the correct explanation of A
(4). A is true but R is false
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
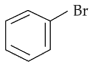
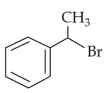
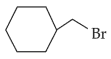
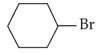
The compound that will undergo \(\text{S}_\text{N}1\) reaction with the fastest rate is:
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The given compound
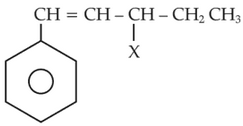
is an example of
(1). aryl halide
(2). allylic halide
(3). vinylic halide
(4). benzylic halide

is an example of
(1). aryl halide
(2). allylic halide
(3). vinylic halide
(4). benzylic halide
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The incorrect statement regarding chirality is
(1). SN1reaction yields 1:1 mixture of bothenantiomers
(2). The product obtained by SN1 reaction ofhaloalkane having chirality at the reactive siteshows inversion of configuration
(3). Enantiomers are superimposable mirror images on each other
(4). A racemic mixture shows zero optical rotation
(1). SN1reaction yields 1:1 mixture of bothenantiomers
(2). The product obtained by SN1 reaction ofhaloalkane having chirality at the reactive siteshows inversion of configuration
(3). Enantiomers are superimposable mirror images on each other
(4). A racemic mixture shows zero optical rotation
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct sequence of bond enthalpy of 'C-X' bond is
(1). CH3-F< CH3-Cl< CH3-Br < CH3-I
(2). CH3-F> CH3-Cl> CH3-Br> CH3-I
(3). CH3-F< CH3-Cl> CH3-Br > CH3-I
(4). CH3-CI> CH3-F>CH3-Br > CH3-I
(1). CH3-F< CH3-Cl< CH3-Br < CH3-I
(2). CH3-F> CH3-Cl> CH3-Br> CH3-I
(3). CH3-F< CH3-Cl> CH3-Br > CH3-I
(4). CH3-CI> CH3-F>CH3-Br > CH3-I
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Elimination reaction of 2-Bromo-pentane to form
pent-2-ene is:
(a) \(\beta\) - Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
Choose the correct option from the following:
(1). (a), (c), (d)
(2). (b), (c), (d)
(3). (a), (b), (d)
(4). (a), (b), (c)
(a) \(\beta\) - Elimination reaction
(b) Follows Zaitsev rule
(c) Dehydrohalogenation reaction
(d) Dehydration reaction
Choose the correct option from the following:
(1). (a), (c), (d)
(2). (b), (c), (d)
(3). (a), (b), (d)
(4). (a), (b), (c)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
ldentify compound X in the following sequence of
reacions:
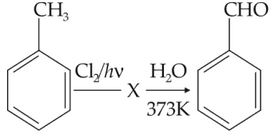
(1).
(2).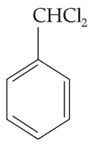
(3).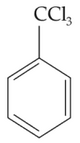
(4).

(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The compound C7H8 undergoes the following reactions:
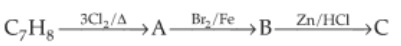
The product 'C' is
(1). p-bromotoluene.
(2). 3-bromo-2,4,6-trichlorotoluene.
(3). o-bromotoluene.
(4). m-bromotoluene.
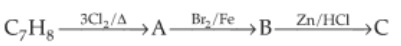
The product 'C' is
(1). p-bromotoluene.
(2). 3-bromo-2,4,6-trichlorotoluene.
(3). o-bromotoluene.
(4). m-bromotoluene.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In the reaction, the electrophile involved is
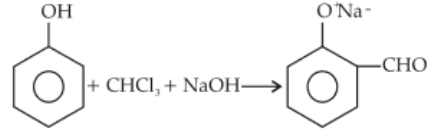
(1). Dichlorocarbene (\(\text{:CCl}_2\))
(2). Dichloromethyl anion (\(\text{C}^+\text{HCl}_2\))
(3). Formyl cation (\(\text{C}^+\text{HO}\)
(4). Dichloromethyl cation (\(\text{C}^+\text{HCl}_2\))
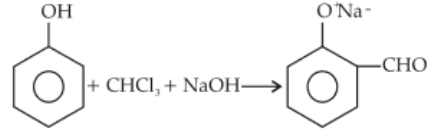
(1). Dichlorocarbene (\(\text{:CCl}_2\))
(2). Dichloromethyl anion (\(\text{C}^+\text{HCl}_2\))
(3). Formyl cation (\(\text{C}^+\text{HO}\)
(4). Dichloromethyl cation (\(\text{C}^+\text{HCl}_2\))
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For the following reactions,
(i) CH3CH2CH2Br + KOH \(\rightarrow\) CH3CH = CH2 + KBr + H2O
(ii)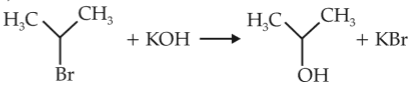
(iii)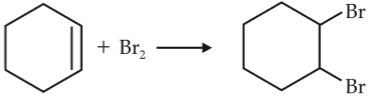
Which of the following statement is correct ?
(1). (i) is elimination reaction, (ii) is substitution and (iii) is addition reaction
(2). (i) is elimination, (ii) and (iii) are substitution reactions
(3). (i) is substitution, (ii) and (iii) are addition reactions
(4). (i) and (ii) are elimination reactions and (iii) is addition reaction
(i) CH3CH2CH2Br + KOH \(\rightarrow\) CH3CH = CH2 + KBr + H2O
(ii)

(iii)

Which of the following statement is correct ?
(1). (i) is elimination reaction, (ii) is substitution and (iii) is addition reaction
(2). (i) is elimination, (ii) and (iii) are substitution reactions
(3). (i) is substitution, (ii) and (iii) are addition reactions
(4). (i) and (ii) are elimination reactions and (iii) is addition reaction
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
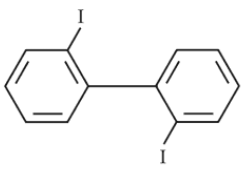
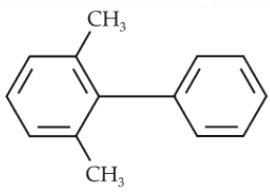
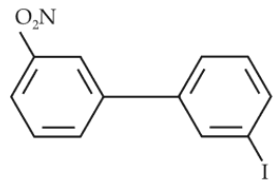
Which of the following biphenyls is optically active ?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
