Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 20
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
If the half-life (\(t_{1/2}\)) for a first order reaction is 1 minute, then the time required for 99.9% completion of the reaction is closest to :
(1). 10 minutes
(2). 2 minutes
(3). 4 minutes
(4). 5 minutes
(1). 10 minutes
(2). 2 minutes
(3). 4 minutes
(4). 5 minutes
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
If the rate constant of a reaction is 0.03 s-1, how much time does it take for 7.2 mol L-1 concentration of the reactant to get reduced to 0.9 mol L-1 ?
(Given: log 2 = 0391)
(1). 21.0 s
(2). 69.3 s
(3). 23.1 s
(4). 210 s
(Given: log 2 = 0391)
(1). 21.0 s
(2). 69.3 s
(3). 23.1 s
(4). 210 s
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The rate of a reaction quadruples where temperature cahnges from \(27^\circ \text{C}\) to \(57^\circ \text{C}\) calculate the energy of activation.
Given \(\text{R}\,=\,8.314\,\text{J}\,\text{K}^{-1}\,\text{mol}^{-1}\), \(\text{log}4\,=\,0.6021\)
(1). \(3.80\, \text{kJ/mol}\)
(2). \(3804\, \text{kJ/mol}\)
(3). \(38.04\, \text{kJ/mol}\)
(4). \(380.4\,\text{ kJ/mol}\)
Given \(\text{R}\,=\,8.314\,\text{J}\,\text{K}^{-1}\,\text{mol}^{-1}\), \(\text{log}4\,=\,0.6021\)
(1). \(3.80\, \text{kJ/mol}\)
(2). \(3804\, \text{kJ/mol}\)
(3). \(38.04\, \text{kJ/mol}\)
(4). \(380.4\,\text{ kJ/mol}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
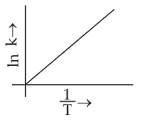
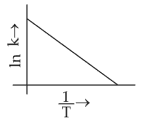
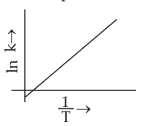
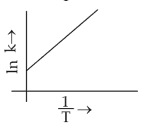
Which plot of \(ln\,k\) vs \(\displaystyle \frac{1}{T}\) is consistent with Arrhenius equation?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Activation energy of any chemical reactions can be calculated if one knows the value of
(1). Orientation of reactant molecules during collision
(2). Rate constant at two different temperatures
(3). Rate constant at standard temepartures
(4). Probability of collision
(1). Orientation of reactant molecules during collision
(2). Rate constant at two different temperatures
(3). Rate constant at standard temepartures
(4). Probability of collision
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For a certain reaction, the rate = k [A]2 [B], when the initial concentration of A is tripled keeping concentration of B constant, the initial rate would
(1). increase by a factor of six
(2). increase by a factor of nine
(3). increase by a factor of three
(4). decrease by a factor of nine
(1). increase by a factor of six
(2). increase by a factor of nine
(3). increase by a factor of three
(4). decrease by a factor of nine
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R :
Assertion A : A reaction can have zero activation energy.
Reason R: The minimum extra amount ofenergy absorbed by reactant molecules so that their enengy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Assertion A : A reaction can have zero activation energy.
Reason R: The minimum extra amount ofenergy absorbed by reactant molecules so that their enengy becomes equal to threshold value, is called activation energy.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The given graph is a representation of kinetics ofa reaction.

The y and x axes for zero and first order reactions,respectively are
(1). zero order (y = concentration and x = time),first order (y = t and x = concentration)
(2). zero order (y = concentration and x = time),first order (y = rate constant and x =concentration)
(3). zero order (y = rate and x = concentration),first order (y = t and x = concentration)
(4). zero order (y = rate and x = concentration),first order (y = rate and x = t)

The y and x axes for zero and first order reactions,respectively are
(1). zero order (y = concentration and x = time),first order (y = t and x = concentration)
(2). zero order (y = concentration and x = time),first order (y = rate constant and x =concentration)
(3). zero order (y = rate and x = concentration),first order (y = t and x = concentration)
(4). zero order (y = rate and x = concentration),first order (y = rate and x = t)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For a first order reaction A → Products, initialconcentration of A is 0.1 M, which becomes0.001 M after 5 minutes. Rate constant for thereaction in min-1 is
(1). 1.3818
(2). 0.9212
(3). 0.4606
(4). 0.2303
(1). 1.3818
(2). 0.9212
(3). 0.4606
(4). 0.2303
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
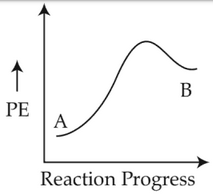
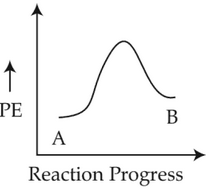
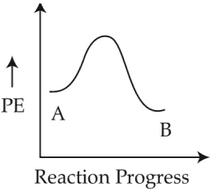
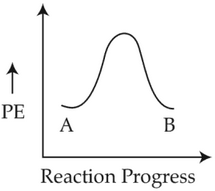
For a reaction A → B, enthalpy of reaction is -4.2 kJ mol-1 and enthalpy of activation is 9.6 kJ mol-1. The correct potential energy profle for the reaction is shown in option.
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The slope of Arrhenius Plot \(\left(\,ln\,k\,v/s\,\frac{1}{T}\right)\) of first order reaction is -5 x 103 K. The value of Ea of the reaction is. Choose the correct option.
[Given R=8.314 JK-1mol-1]
(1). 41.5 kJ mol-1
(2). 83.0 kJ mol-1
(3). 166 kJ mol-1
(4). -83 kJ mol-1
[Given R=8.314 JK-1mol-1]
(1). 41.5 kJ mol-1
(2). 83.0 kJ mol-1
(3). 166 kJ mol-1
(4). -83 kJ mol-1
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The rate constant for a first order reaction is
4.606 x 10-3s-1, The time required to reduce 2.0g of the reactant to 0.2 g is:
(1). 200 s
(2). 500 s
(3). 1000 s
(4). 100 s
(1). 200 s
(2). 500 s
(3). 1000 s
(4). 100 s
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
If the rate constant for a first order reaction is k, the time (t) required for the completion of 99% of the reaction is given by
(1). t = 6.909/k
(2). t = 4.606/k
(3). t = 2.303/k
(4). t = 0.693/k
(1). t = 6.909/k
(2). t = 4.606/k
(3). t = 2.303/k
(4). t = 0.693/k
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
When initial concentration of the reactant is doubled, the half-life period of a zero order reaction
(1). remains unchanged
(2). is tripled
(3). is doubled
(4). is halved
(1). remains unchanged
(2). is tripled
(3). is doubled
(4). is halved
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct difference between first and second order reactions is that
(1). the rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations.
(2). a first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed.
(3). the half-life of a first-order reaction does not depend on [A]0 ; the half-life of a second-order reaction does depend on [A]0 .
(4). the rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations.
(1). the rate of a first-order reaction does depend on reactant concentrations; the rate of a second-order reaction does not depend on reactant concentrations.
(2). a first-order reaction can be catalyzed; a second-order reaction cannot be catalyzed.
(3). the half-life of a first-order reaction does not depend on [A]0 ; the half-life of a second-order reaction does depend on [A]0 .
(4). the rate of a first-order reaction does not depend on reactant concentrations; the rate of a second-order reaction does depend on reactant concentrations.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Mechanism of a hypothetical reaction X2 + Y2 \(\rightarrow\) 2XY is given below:
(i) X2 \(\rightarrow\) X + X (fast)
(ii) X + Y2 \(\rightleftharpoons\) XY + Y (slow)
(iii) X + Y \(\rightarrow\) XY (fast)
The overall order of the reaction will be
(1). 1.5
(2). 1
(3). 2
(4). 0
(i) X2 \(\rightarrow\) X + X (fast)
(ii) X + Y2 \(\rightleftharpoons\) XY + Y (slow)
(iii) X + Y \(\rightarrow\) XY (fast)
The overall order of the reaction will be
(1). 1.5
(2). 1
(3). 2
(4). 0
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A first order reaction has a specific reaction rate of 10-2 s-1. How much time will it take for 20 g of the reactant to reduce to 5 g ?
(1). 693.0 second
(2). 238.6 second
(3). 138.6 second
(4). 346.5 second
(1). 693.0 second
(2). 238.6 second
(3). 138.6 second
(4). 346.5 second
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the nitration of benzene using mixed conc . H2SO4 and HNO3 . If a large amount of KHSO4 is added to the mixture , the rate of nitration will be
(1). slower
(2). unchanged
(3). doubled
(4). faster
(1). slower
(2). unchanged
(3). doubled
(4). faster
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The rate of a first - order reaction is 0.04 mol L-1S-1 at 10 sec and 0.03 mol L-1S-1 at 20 sec after initiation of the reaction . The half - life period of the reaction is
(1). 34.1 s
(2). 44.1 s
(3). 54.1 s
(4). 24.1 s
(1). 34.1 s
(2). 44.1 s
(3). 54.1 s
(4). 24.1 s
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The addition of a catalyst during a chemical reaction alters which of the following quantities ?
(1). Internal energy
(2). Enthalpy
(3). Activation energy
(4). Entropy
(1). Internal energy
(2). Enthalpy
(3). Activation energy
(4). Entropy
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
