Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 22
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
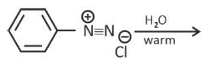
Which one of the following reactions does
NOT give benzene as the product ?
(1).
(2).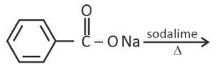
(3).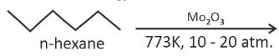
(4).
(1).

(2).
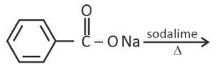
(3).
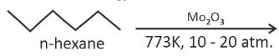
(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
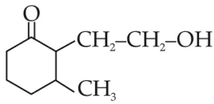
The product formed from the following chemical reaction is
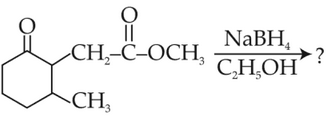
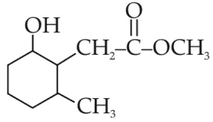
(1).
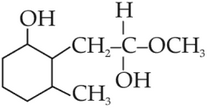
(2).
(3).
(4).
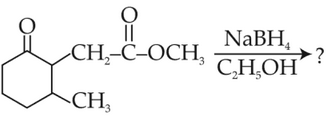
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following diatomic molecular species has only π bonds according of Molecular Orbital Theory ?
(1). N2
(2). C2
(3). Be2
(4). O3
(1). N2
(2). C2
(3). Be2
(4). O3
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
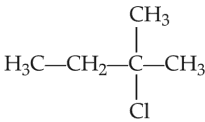
An alkene ‘A’ on reaction with O3 and Zn-H2O gives propanone and ethanal in equimolar and ratio. Addition of HCl to alkene ‘A’ gives ‘B’ as the major product. The structure of product ‘B’ is
(1).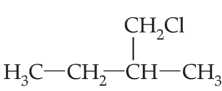
(2).
(3).
(4).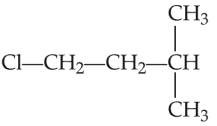
(1).
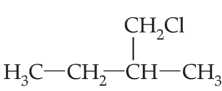
(2).

(3).

(4).
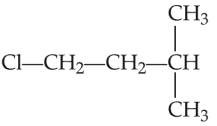
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List I with List II
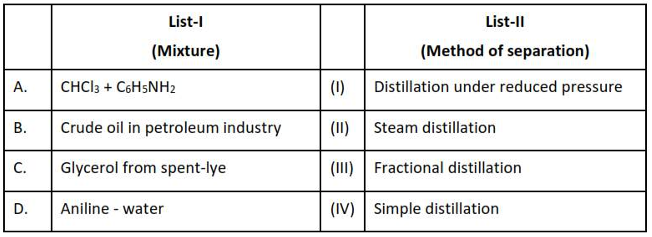
Choose the correct answer from the options given below :
(1). A-II, B-IV, C-II, D-I
(2). A-IV, B-III, С-I, D-II
(3). A-IV, B-III, C-II, D-I
(4). A-III, B-IV, C-I, D-II

Choose the correct answer from the options given below :
(1). A-II, B-IV, C-II, D-I
(2). A-IV, B-III, С-I, D-II
(3). A-IV, B-III, C-II, D-I
(4). A-III, B-IV, C-I, D-II
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
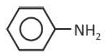
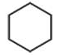
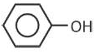
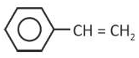
Which one of the following compounds does not decolourize bromine water?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
How many products (including stereo isomers) are expected from monochlorination of the following compound?
(1). 6
(2). 2
(3). 3
(4). 5

(1). 6
(2). 2
(3). 3
(4). 5
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
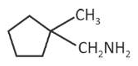
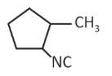
predict the major product 'P' in the following sequence of reactions -
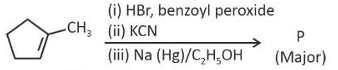
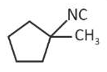
(1).
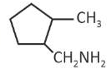
(2).
(3).
(4).
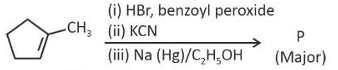
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List-I with List-II:
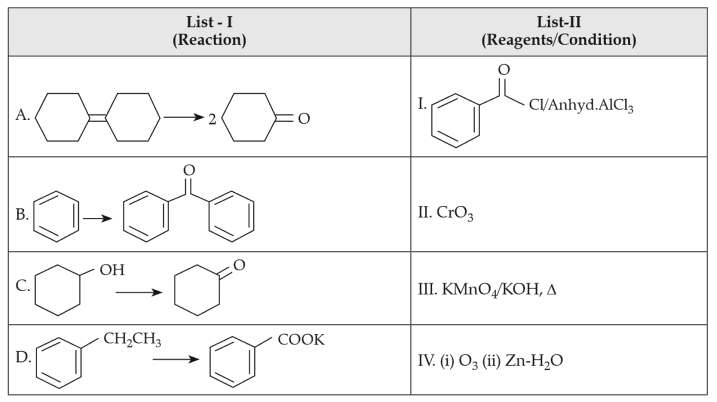
(1). A-IV, B-I, C-II, D-III
(2). A-I, B-IV, C-II, D-III
(3). A-IV, B-I, C-III, D-II
(4). A-III, B-I, C-II, D-IV

(1). A-IV, B-I, C-II, D-III
(2). A-I, B-IV, C-II, D-III
(3). A-IV, B-I, C-III, D-II
(4). A-III, B-I, C-II, D-IV
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements:
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
(1). Statement I is correct but Statement II is incorrect
(2). Statement I is incorrect but Statement II is correct
(3). Both Statement I and Statement II are correct
(4). Both Statement I and Statement II are incorrect
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
(1). Statement I is correct but Statement II is incorrect
(2). Statement I is incorrect but Statement II is correct
(3). Both Statement I and Statement II are correct
(4). Both Statement I and Statement II are incorrect
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Identify the correct reagents that would bring about the following transformation.
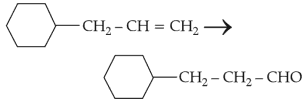
(1).
(2).
(3).
(4).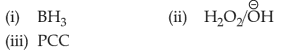

(1).

(2).

(3).

(4).
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
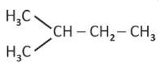
A compound with a molecular formula of \(\text{C}_6\text{H}_{14}\) has two tertiary carbons. Its IUPAC name is:
(1). 2,3-dimethylbutane
(2). 2,2-dimethylybutane
(3). n-hexane
(4). 2-methylpentane
(1). 2,3-dimethylbutane
(2). 2,2-dimethylybutane
(3). n-hexane
(4). 2-methylpentane
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Weight (g) of two moles of the organic compound,
which is obtained by heating sodium ethanoate
with sodium hydroxide in presence of calcium
Oxide is:
(1). 32
(2). 30
(3). 18
(4). 16
(1). 32
(2). 30
(3). 18
(4). 16
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The major product formed in dehydrohalogenation reaction of 2-Bromo pentane is Pent-2-ene. This product formation is based on?
(1). Saytzeff's Rule
(2). Hund's Rule
(3). Hoffmann Rule
(4). Huckel's Rule
(1). Saytzeff's Rule
(2). Hund's Rule
(3). Hoffmann Rule
(4). Huckel's Rule
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The major product of the following chemical
reaction is:
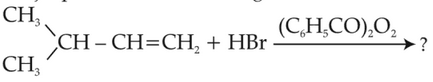
(1).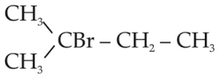
(2).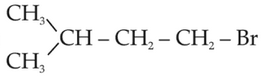
(3).
(4).
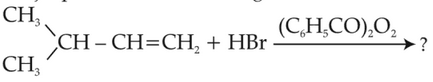
(1).
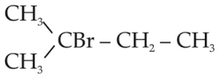
(2).
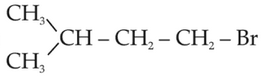
(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List-I with List-II
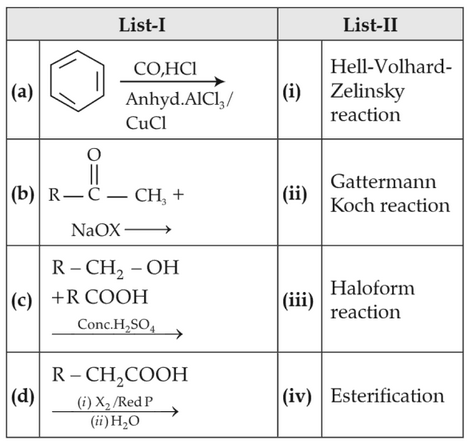
Choose the correct answer from the options given below.
(1). (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
(2). (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
(3). (a)-(i), (b)-(iv), (c)-(iii), (d)-(ii)
(4). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)

Choose the correct answer from the options given below.
(1). (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
(2). (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
(3). (a)-(i), (b)-(iv), (c)-(iii), (d)-(ii)
(4). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The most suitable reagent for the following conversion is
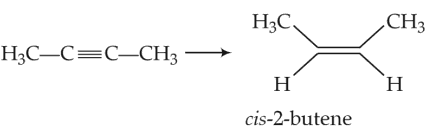
(1). H2, Pd/C, quinoline
(2). Zn/HCl
(3). Hg2+/H+, H2O
(4). Na/Liquid NH3

(1). H2, Pd/C, quinoline
(2). Zn/HCl
(3). Hg2+/H+, H2O
(4). Na/Liquid NH3
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
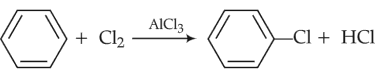
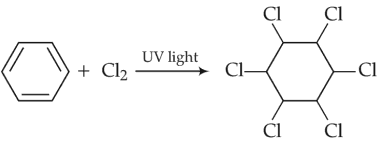
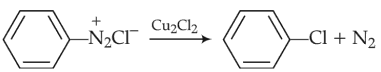
Among the following, the reaction that proceeds through an electrophilic substitution, is
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Hydrocarbon (A) reacts with bromine by substitution to form an alkyl bromide which by Wurtz reaction is converted to gaseous hydrocarbon containing less than four carbon atoms. (A) is
(1). CH4
(2). CH3 – CH3
(3). CH2 = CH2
(4). CH ≡ CH
(1). CH4
(2). CH3 – CH3
(3). CH2 = CH2
(4). CH ≡ CH
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Predict the correct intermediate and product in the following reaction
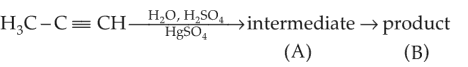
(1).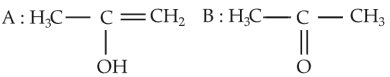
(2).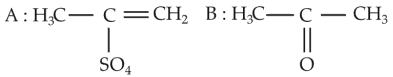
(3).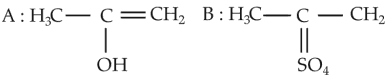
(4).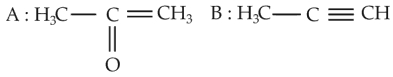
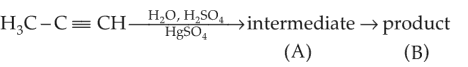
(1).
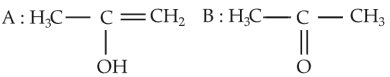
(2).
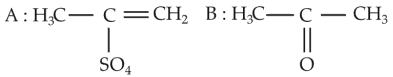
(3).
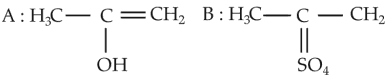
(4).
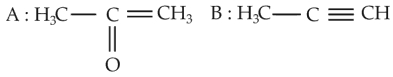
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one is the correct order of acidity ?
(1). CH3 – CH3 > CH2 = CH2 > CH3 –C ≡ CH > CH ≡ CH
(2). CH2 = CH2 > CH3 – CH = CH2 > CH3 – C ≡ CH
(3). CH ≡ CH > CH3 – C ≡ CH > CH2 = CH2 > CH3 – - CH3
(4). CH ≡ CH > CH2 = CH2 > CH3 – C ≡ CH > CH3 – CH3
(1). CH3 – CH3 > CH2 = CH2 > CH3 –C ≡ CH > CH ≡ CH
(2). CH2 = CH2 > CH3 – CH = CH2 > CH3 – C ≡ CH
(3). CH ≡ CH > CH3 – C ≡ CH > CH2 = CH2 > CH3 – - CH3
(4). CH ≡ CH > CH2 = CH2 > CH3 – C ≡ CH > CH3 – CH3
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In the reaction,
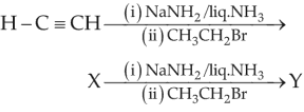
(1). X = 2-butyne ; Y = 3-hexyne
(2). X = 2-butyne ; Y = 2-hexyne
(3). X = 1-butyne ; Y = 2-hexyne
(4). X = 1-butyne ; Y = 3-hexyne
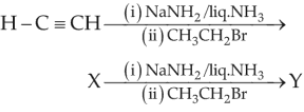
(1). X = 2-butyne ; Y = 3-hexyne
(2). X = 2-butyne ; Y = 2-hexyne
(3). X = 1-butyne ; Y = 2-hexyne
(4). X = 1-butyne ; Y = 3-hexyne
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
