Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 28
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
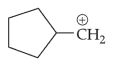
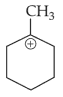
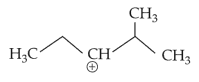
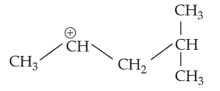
The most stable carbocation among the following is:
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
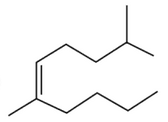
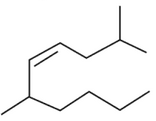
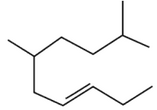
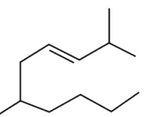
The correct structure of 2, 6-Dimethyl-dec-4-ene
is:
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
What is the IUPAC name of the organic compound formed in the following chemical reaction ?
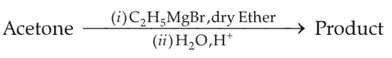
(1). 2-methyl propan-2-ol
(2). pentan-2-ol
(3). pentan-3-ol
(4). 2-methyl butan-2-ol
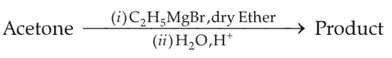
(1). 2-methyl propan-2-ol
(2). pentan-2-ol
(3). pentan-3-ol
(4). 2-methyl butan-2-ol
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
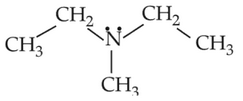
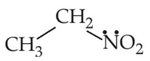
Identify the compound that will react with Hinsberg's reagent to give a solid which dissolves in alkali:
(1).
(2).
(3).
(4).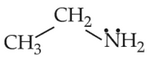
(1).

(2).

(3).

(4).
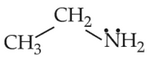
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the following compounds can exist as cis-trans isomers?
(1). 1,2-Dimethylcyclohexane
(2). Pent-1-ene
(3). 2-Methylhex-2-ene
(4). 1,1-Dimethylcyclopropane
(1). 1,2-Dimethylcyclohexane
(2). Pent-1-ene
(3). 2-Methylhex-2-ene
(4). 1,1-Dimethylcyclopropane
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
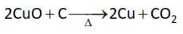
Which one of the following reactions does
NOT belong to "Lassaigne's test"?
(1).
(2).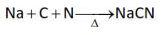
(3).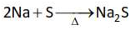
(4).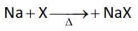
(1).

(2).
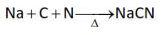
(3).
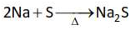
(4).
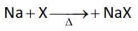
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
On heating, some solid substances change from solid to vapour state without passing through liquid state. The technique used for the purification of such solid substances based on the above principle is konwon as
(1). Distillation
(2). Chromatography
(3). Crystallization
(4). Sublimation
(1). Distillation
(2). Chromatography
(3). Crystallization
(4). Sublimation
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In Lassaigne's extract of an organic compound, both nitrogen and sulphur are present, which gives blood red colour with Fe3+ due to the formation of
(1). NaSCN
(2). [Fe(CN)5NOS]4-
(3). [Fe(SCN)]2+
(4). Fe[Fe(CN)6]3 . xH2O
(1). NaSCN
(2). [Fe(CN)5NOS]4-
(3). [Fe(SCN)]2+
(4). Fe[Fe(CN)6]3 . xH2O
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
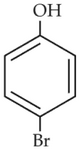
Identify the product in the following reaction:
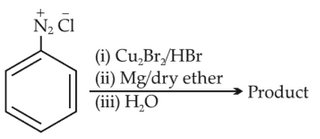
(1).
(2).
(3).
(4).
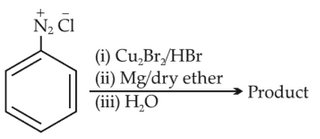
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the following compounds/species.
The number of compounds/species which obey Huckel's rule is
(1). 6
(2). 2
(3). 5
(4). 4
The number of compounds/species which obey Huckel's rule is
(1). 6
(2). 2
(3). 5
(4). 4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
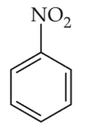
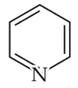
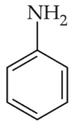
The Kjeldahl's method for the estimation of nitrogen can be used to estimate the amount of nitrogen in which one of the following compounds?
(1).
(2).
(3).
(4).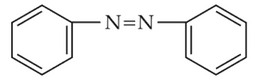
(1).

(2).

(3).

(4).
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
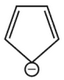
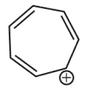
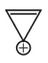
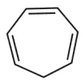
Which compound amongst the following is not anaromatic compound?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
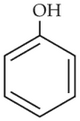
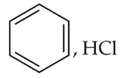
Which of the following sequence of reactions issuitable to synthesize chlorobenzene?
(1). Benzene, Cl2, anhydrous FeCl3
(2). Phenol, NaNO2, HCl, CuCl
(3).
(4).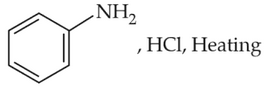
(1). Benzene, Cl2, anhydrous FeCl3
(2). Phenol, NaNO2, HCl, CuCl
(3).

(4).
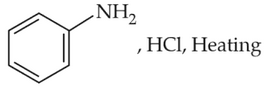
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct IUPAC name of the following compound is
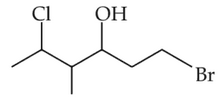
(1). 1-bromo-5-chloro-4-methylhexan-3-ol
(2). 6-bromo-2-chloro-4-methythexan-4-ol
(3). 1-bromo-4-methyl-5-chlorohexan-3-ol
(4). 6-bromo-4-methyl-2-chlorohexan-4-ol

(1). 1-bromo-5-chloro-4-methylhexan-3-ol
(2). 6-bromo-2-chloro-4-methythexan-4-ol
(3). 1-bromo-4-methyl-5-chlorohexan-3-ol
(4). 6-bromo-4-methyl-2-chlorohexan-4-ol
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Compound X on reaction with O3 followed by Zn/H2O gives formaldehyde and 2-methyl propanal as products.
The compound X is
(1). 3-Methylbut-1-ene
(2). 2-Methylbut-1-ene
(3). 2-Methylbut-2-ene
(4). Pent-2-ene
The compound X is
(1). 3-Methylbut-1-ene
(2). 2-Methylbut-1-ene
(3). 2-Methylbut-2-ene
(4). Pent-2-ene
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Dihedral angle of least stable conformer of ethane
is:
(1). 120°
(2). 180°
(3). 60°
(4). 0°
(1). 120°
(2). 180°
(3). 60°
(4). 0°
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
An organic compound contains 78% (by wt.) carbon and remaining percentage of hydrogen. The right option for the empirical formula of this compound is [Atomic wt. of C is 12, H is 1]
(1). CH
(2). CH2
(3). CH3
(4). CH4
(1). CH
(2). CH2
(3). CH3
(4). CH4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The compound which shows metamerism is :
(1). C5H12
(2). C3H8O
(3). C3H6O
(4). C4H10O
(1). C5H12
(2). C3H8O
(3). C3H6O
(4). C4H10O
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
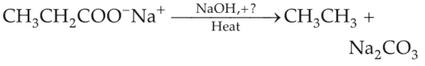
Consider the above reaction and identify the missing reagent/chemical.
(1). B2H6
(2). Red Phosphorus
(3). CaO
(4). DIBAL-H
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The reagent 'R' in the given sequence of chemical reaction is
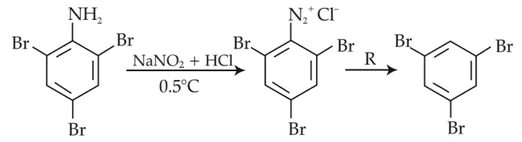
(1). H2O
(2). CH3CH2OH
(3). HI
(4). CuCN/KCN

(1). H2O
(2). CH3CH2OH
(3). HI
(4). CuCN/KCN
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
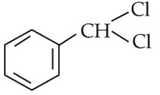
The intermediate compound "X" in the following chemical reaction is :
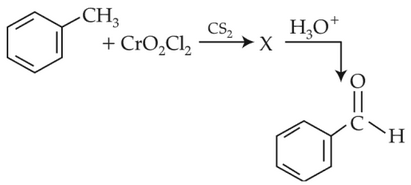
(1).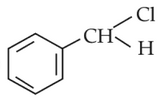
(2).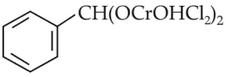
(3).
(4).

(1).

(2).
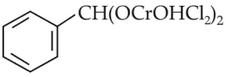
(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A tertiary butyl carbocation is more stable than a secondary butyl carbocation because of which of
the following ?
(1). +R effect of -CH groups
(2). -R effect of -CH groups
(3). Hyperconjugation
(4). -I effect of -CH3 groups
(1). +R effect of -CH
(2). -R effect of -CH
(3). Hyperconjugation
(4). -I effect of -CH3 groups
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A mixture of 2.3 g formic acid and 4.5 g oxalic acid is treated with conc. H2SO4. The evolved gaseous mixture is passed through KOH pellets. Weight (in g) of the remaining product at STP will be
(1). 4.4
(2). 2.8
(3). 3.0
(4). 1.4
(1). 4.4
(2). 2.8
(3). 3.0
(4). 1.4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
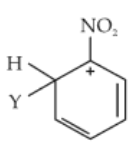
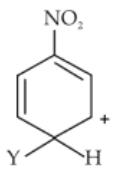
Which of the following carbocations is expected to be most stable ?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following is correct with respect to -1 effect of yje substituents? (R = alkyl)
(1). -NR2 > -OR > -F
(2). -NH2 > _OR > -F
(3). -NR2 < -OR < -F
(4). -NH2 < _OR < -F
(1). -NR2 > -OR > -F
(2). -NH2 > _OR > -F
(3). -NR2 < -OR < -F
(4). -NH2 < _OR < -F
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The IUPAC name of the compound is
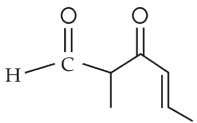
(1). 3-keto-2-methylhex-5-enal
(2). 3-keto-2-methylhex-4-enal
(3). 5-formylhex-2-en-3-one
(4). 5-methyl-4-oxohex-2-en-5-al

(1). 3-keto-2-methylhex-5-enal
(2). 3-keto-2-methylhex-4-enal
(3). 5-formylhex-2-en-3-one
(4). 5-methyl-4-oxohex-2-en-5-al
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct statement regarding electrophile is
(1). Electrophile can be either neutral or positively charged species and can form a bond by accepting a pair of electrons from a nucleophile.
(2). Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile
(3). Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electro-phile
(4). Electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile
(1). Electrophile can be either neutral or positively charged species and can form a bond by accepting a pair of electrons from a nucleophile.
(2). Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile
(3). Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electro-phile
(4). Electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct statement regarding the comparison of staggered and eclipsed conformations of ethane, is
(1). The eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has no torsional strain
(2). The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain
(3). The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain
(4). The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain
(1). The eclipsed conformation of ethane is more stable than staggered conformation, because eclipsed conformation has no torsional strain
(2). The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has torsional strain
(3). The staggered conformation of ethane is more stable than eclipsed conformation, because staggered conformation has no torsional strain
(4). The staggered conformation of ethane is less stable than eclipsed conformation, because staggered conformation has torsional strain
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
