Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 12
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
What is the change in oxidation number of carbon in the following reaction ?
\(\text{CH}_4\text{(g)} \,+\, \text{4Cl}_2\text{(g)} \, \rightarrow \, \text{CCl}_4\text{(i)}\, +\, \text{4HCl(g)}\)
(1). 0 to +4
(2). -4 to +4
(3). 0 to -4
(4). +4 to +4
\(\text{CH}_4\text{(g)} \,+\, \text{4Cl}_2\text{(g)} \, \rightarrow \, \text{CCl}_4\text{(i)}\, +\, \text{4HCl(g)}\)
(1). 0 to +4
(2). -4 to +4
(3). 0 to -4
(4). +4 to +4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the following compounds:
\(\text{K0}_2\), \(\text{H}_2\text{0}_2\) and \(\text{H}_2\text{SO}_4\)
The oxidation states of the underlined elements in them are, respectively,
(1). +4, -4, and +6
(2). +1, -1, and +6
(3). +2, -2, and +6
(4). +1, -2, and +4
\(\text{K0}_2\), \(\text{H}_2\text{0}_2\) and \(\text{H}_2\text{SO}_4\)
The oxidation states of the underlined elements in them are, respectively,
(1). +4, -4, and +6
(2). +1, -1, and +6
(3). +2, -2, and +6
(4). +1, -2, and +4
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Among group 16 elements, which one does NOT show -2 oxidation state
(1). Te
(2). Po
(3). O
(4). Se
(1). Te
(2). Po
(3). O
(4). Se
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which is NOT a redox reaction?
(1). \(\text{H}_2\,+\,\text{Cl}_2\,\rightarrow\,2\text{HCl}\)
(2). \(\text{BaCl}_2\,+\,\text{Na}_2\text{SO}_4\,\rightarrow\,\text{BaSO}_4\,+\,2\text{NaCl}\)
(3). \(\text{Zn}\,+\,\text{CuSO}_4\,\rightarrow\,\text{ZnSO}_4\,+\,\text{Cu}\)
(4). \(2\text{KClO}_3\,+\,\text{I}_2\,\rightarrow\,2\text{KIO}_3\,+\,\text{Cl}_2\)
(1). \(\text{H}_2\,+\,\text{Cl}_2\,\rightarrow\,2\text{HCl}\)
(2). \(\text{BaCl}_2\,+\,\text{Na}_2\text{SO}_4\,\rightarrow\,\text{BaSO}_4\,+\,2\text{NaCl}\)
(3). \(\text{Zn}\,+\,\text{CuSO}_4\,\rightarrow\,\text{ZnSO}_4\,+\,\text{Cu}\)
(4). \(2\text{KClO}_3\,+\,\text{I}_2\,\rightarrow\,2\text{KIO}_3\,+\,\text{Cl}_2\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
On balancing the given redox reaction,
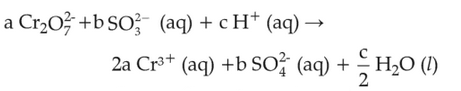
the coefficients a, b and c are found to be, respectively
(1). 3, 8, 1
(2). 1, 8, 3
(3). 8, 1, 3
(4). 1, 3, 8
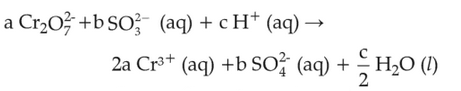
the coefficients a, b and c are found to be, respectively
(1). 3, 8, 1
(2). 1, 8, 3
(3). 8, 1, 3
(4). 1, 3, 8
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following reactions is the metal displacement reaction? Choose the right option.
(1).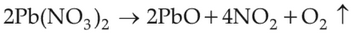
(2).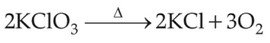
(3).
(4).
(1).
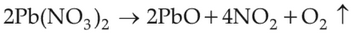
(2).
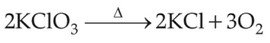
(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following reactions are disproportionation reaction ?
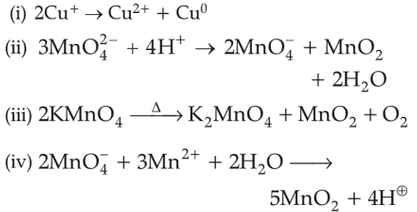
Select the correct option from the following:
(1). (i), (ii), and (iii)
(2). (i), (iii), and (iv)
(3). (i) and (iv) only
(4). (i) and (ii) only
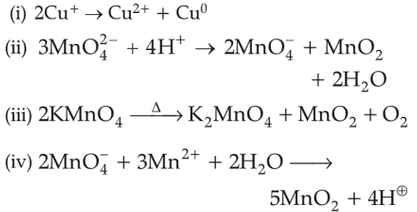
Select the correct option from the following:
(1). (i), (ii), and (iii)
(2). (i), (iii), and (iv)
(3). (i) and (iv) only
(4). (i) and (ii) only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below
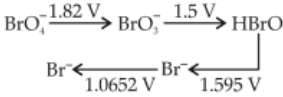
Then the species undergoing dispro-portionation is
(1). \(\text{HB}_rO\)
(2). \(\text{Br}_2\)
(3). \(\text{BrO}_4^-\)
(4). \(\text{BrO}_3^-\)
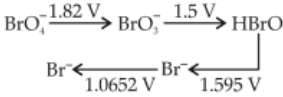
Then the species undergoing dispro-portionation is
(1). \(\text{HB}_rO\)
(2). \(\text{Br}_2\)
(3). \(\text{BrO}_4^-\)
(4). \(\text{BrO}_3^-\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The correct order of N-compounds in its decreasing order of oxidation states is
(1). NH4Cl, N2, NO, HNO3
(2). HNO3, NH4Cl, NO, N2
(3). HNO3, NO, NH4Cl, N2
(4). HNO3 , NO, N2 , NH4Cl
(1). NH4Cl, N2, NO, HNO3
(2). HNO3, NH4Cl, NO, N2
(3). HNO3, NO, NH4Cl, N2
(4). HNO3 , NO, N2 , NH4Cl
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For the redox reaction
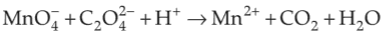
The correct coefficients of the reactants for the balanced equation are
(1). \(\text{MNO}_4^- \rightarrow \) 5, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 16, \(\text{H1}^+ \rightarrow \) 2
(2). \(\text{MNO}_4^- \rightarrow \) 2, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 16, \(\text{H1}^+ \rightarrow \) 5
(3). \(\text{MNO}_4^- \rightarrow \) 2, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 5, \(\text{H1}^+ \rightarrow \) 16
(4). \(\text{MNO}_4^- \rightarrow \) 16, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 5, \(\text{H1}^+ \rightarrow \) 2
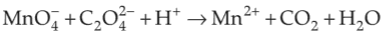
The correct coefficients of the reactants for the balanced equation are
(1). \(\text{MNO}_4^- \rightarrow \) 5, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 16, \(\text{H1}^+ \rightarrow \) 2
(2). \(\text{MNO}_4^- \rightarrow \) 2, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 16, \(\text{H1}^+ \rightarrow \) 5
(3). \(\text{MNO}_4^- \rightarrow \) 2, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 5, \(\text{H1}^+ \rightarrow \) 16
(4). \(\text{MNO}_4^- \rightarrow \) 16, \(\text{C}_2 \text{O}_4^{2-} \rightarrow \) 5, \(\text{H1}^+ \rightarrow \) 2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Name the gas that can readily decolourizes acidified KMnO4 solution.
(1). P2O5
(2). CO2
(3). SO2
(4). NO2
(1). P2O5
(2). CO2
(3). SO2
(4). NO2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which pair of ions both the species contain S – S bond ?
(1). \(\text{S}_4 O_6^{2-}\), \(\text{S}_2 O_7^{2-}\)
(2). \(\text{S}_2 O_7^{2-}\), \(\text{S}_2 O_3^{2-}\)
(3). \(\text{S}_4 O_6^{2-}\), \(\text{S}_2 O_3^{2-}\)
(4). \(\text{S}_2 O_7^{2-}\), \(\text{S}_2 O_8^{2-}\)
(1). \(\text{S}_4 O_6^{2-}\), \(\text{S}_2 O_7^{2-}\)
(2). \(\text{S}_2 O_7^{2-}\), \(\text{S}_2 O_3^{2-}\)
(3). \(\text{S}_4 O_6^{2-}\), \(\text{S}_2 O_3^{2-}\)
(4). \(\text{S}_2 O_7^{2-}\), \(\text{S}_2 O_8^{2-}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
