Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 28
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
For the reaction \(A\left(g\right) \rightleftharpoons 2B\left(g\right)\), the backward reaction rate constant is higher than the forward reaction rate constant by a factor of 2500, at 1000 K.
[Given : R = 0.0831 L atm mol-1 K-1]Kp for the reaction at 1000 K is
(1). 0.021
(2). 83.1
(3). 2.077 × 105
(4). 0.033
[Given : R = 0.0831 L atm mol-1 K-1]Kp for the reaction at 1000 K is
(1). 0.021
(2). 83.1
(3). 2.077 × 105
(4). 0.033
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Conjugate base for Bronsted acids H2O and HF are
(1). H3O+ and F– , respectively.
(2). OH– and F–, respectively.
(3). H3O+ and H2F+, respectively.
(4). OH– and H2F+, respectively.
(1). H3O+ and F– , respectively.
(2). OH– and F–, respectively.
(3). H3O+ and H2F+, respectively.
(4). OH– and H2F+, respectively.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
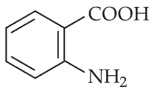
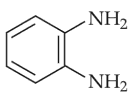
The major product of the following reaction is
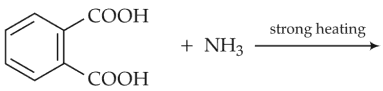
(1).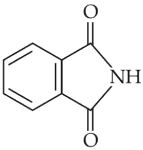
(2).
(3).
(4).
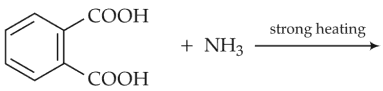
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
MY and NY3, two nearly insoluble salts have the same Ksp values of 6.2 × 10-13 at room temperature. Which statement would be true in regard to MY and NY3 ?
(1). The molar solubility of MY in water is less than of NY3
(2). The salts MY and NY3 are more soluble in 0.5 M KY than in pure water
(3). The addition of the salt of KY to solution of MY and NY3 will have no effect on their solubilities
(4). The molar solubilities of MY and NY3 in water are identical
(1). The molar solubility of MY in water is less than of NY3
(2). The salts MY and NY3 are more soluble in 0.5 M KY than in pure water
(3). The addition of the salt of KY to solution of MY and NY3 will have no effect on their solubilities
(4). The molar solubilities of MY and NY3 in water are identical
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Phosphoric acid ionizes in three steps with their ionization constant values
\(Ka_1\), \(Ka_2\), and \(Ka_3\), respectively,while K is the overall ionization constant.
Which of the following statements are true?
A. \(\text{log} K\) = \(\text{log} Ka_1\) + \(\text{log}Ka_2\) +\(\text{log}Ka_3\)
B. \(H_3PO_4\) is a stronger acid than \(H_2PO_4^-\) and \(HPO_4^{2-}\).
С. \(Ka_1\) > \(Ka_2\) > \(Ka_3\).
D. \(\displaystyle Ka_1\,= \,\frac{Ka_3\,+\,Ka_2}{2}\).
Choose the correct answer from the options given below:
(1). A, B and C only
(2). A and B only
(3). A and C only
(4). B, C and D only
\(Ka_1\), \(Ka_2\), and \(Ka_3\), respectively,while K is the overall ionization constant.
Which of the following statements are true?
A. \(\text{log} K\) = \(\text{log} Ka_1\) + \(\text{log}Ka_2\) +\(\text{log}Ka_3\)
B. \(H_3PO_4\) is a stronger acid than \(H_2PO_4^-\) and \(HPO_4^{2-}\).
С. \(Ka_1\) > \(Ka_2\) > \(Ka_3\).
D. \(\displaystyle Ka_1\,= \,\frac{Ka_3\,+\,Ka_2}{2}\).
Choose the correct answer from the options given below:
(1). A, B and C only
(2). A and B only
(3). A and C only
(4). B, C and D only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Higher yield of NO in
\(N_2\left(8\right)\, +\, O_2\left(g\right)\, \rightleftharpoons\, 2NO\left(g\right)\) can be obtained at
[\(\Delta H\) of the reaction = +180.7 kJ mol-1]
A. higher temperature
B. lower temperature
C. higher concentration of N2
D. higher concentration of O2
Choose the correct answer from the options given below:
(1). A, C, D only
(2). A, D only
(3). B, C only
(4). B, C, D only
\(N_2\left(8\right)\, +\, O_2\left(g\right)\, \rightleftharpoons\, 2NO\left(g\right)\) can be obtained at
[\(\Delta H\) of the reaction = +180.7 kJ mol-1]
A. higher temperature
B. lower temperature
C. higher concentration of N2
D. higher concentration of O2
Choose the correct answer from the options given below:
(1). A, C, D only
(2). A, D only
(3). B, C only
(4). B, C, D only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For the reaction \(2A \rightleftharpoons B + C, \, K_c \, = \, 4 \times 10^{-3}\). At a given time, the composition of reaction mixture is:
\([A]\, =\, [B]\, = \, [C]\, =\, 2 \times 10^{-3}\,M\)
Then, which of the following is correct?
(1). Reaction has a tendency to go in backward direction.
(2). Reaction has gone to completion in forward direction.
(3). Reaction is at eqilibrium.
(4). Reaction has a tendency to go in forward direction.
\([A]\, =\, [B]\, = \, [C]\, =\, 2 \times 10^{-3}\,M\)
Then, which of the following is correct?
(1). Reaction has a tendency to go in backward direction.
(2). Reaction has gone to completion in forward direction.
(3). Reaction is at eqilibrium.
(4). Reaction has a tendency to go in forward direction.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which of the following equilibria, \(\text{K}_\text{P}\) and \(\text{K}_\text{C}\) are NOT equal?
(1). \(\text{CO}\left(\text{g}\right)\,+\,\text{H}_2\text{O}\left(\text{g}\right) \rightleftharpoons \text{CO}_2\left(\text{g}\right)\, +\, \text{H}_2\left(\text{g}\right)\)
(2). \(2\text{BrCl}\left(\text{g}\right) \rightleftharpoons \text{Br}_2\left(\text{g}\right)\, +\, \text{Cl}_2\left(\text{g}\right)\)
(3). \(\text{PCl}_5\left(\text{g}\right) \rightleftharpoons \text{PCl}_3\left(\text{g}\right)\, +\, \text{Cl}_2\left(\text{g}\right)\)
(4). \(\text{H}_2\left(\text{g}\right)\,+\,\text{I}_2\left(\text{g}\right) \rightleftharpoons 2\text{HI}\left(\text{g}\right)\)
(1). \(\text{CO}\left(\text{g}\right)\,+\,\text{H}_2\text{O}\left(\text{g}\right) \rightleftharpoons \text{CO}_2\left(\text{g}\right)\, +\, \text{H}_2\left(\text{g}\right)\)
(2). \(2\text{BrCl}\left(\text{g}\right) \rightleftharpoons \text{Br}_2\left(\text{g}\right)\, +\, \text{Cl}_2\left(\text{g}\right)\)
(3). \(\text{PCl}_5\left(\text{g}\right) \rightleftharpoons \text{PCl}_3\left(\text{g}\right)\, +\, \text{Cl}_2\left(\text{g}\right)\)
(4). \(\text{H}_2\left(\text{g}\right)\,+\,\text{I}_2\left(\text{g}\right) \rightleftharpoons 2\text{HI}\left(\text{g}\right)\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Consider the following reaction in a sealed vessel at equilibrium with concentrations of
\(\text{N}_2\,=\,3.0 \times 10^{-3}\text{M}\),
\(\text{O}_2\,=\,4.2 \times 10^{-3}\text{M}\),
\(\text{NO}\,=\,2.8 \times 10^{-3}\text{M}\),
\(2\text{NO}\left(g\right)\,\rightleftharpoons\, \text{N}_2\left(g\right)\,+\, \text{O}_2\left(g\right)\),
If \( 0.1\text{molL}^{-1}\) of \(\text{NO}\left(g\right)\) is taken in a closed vessel, what will be degree of dissociation \(\left(\alpha\right)\) of \(\text{NO}\left(g\right)\) at equilibrium?
(1). 0.8889
(2). 0.717
(3). 0.00889
(4). 0.0889
\(\text{N}_2\,=\,3.0 \times 10^{-3}\text{M}\),
\(\text{O}_2\,=\,4.2 \times 10^{-3}\text{M}\),
\(\text{NO}\,=\,2.8 \times 10^{-3}\text{M}\),
\(2\text{NO}\left(g\right)\,\rightleftharpoons\, \text{N}_2\left(g\right)\,+\, \text{O}_2\left(g\right)\),
If \( 0.1\text{molL}^{-1}\) of \(\text{NO}\left(g\right)\) is taken in a closed vessel, what will be degree of dissociation \(\left(\alpha\right)\) of \(\text{NO}\left(g\right)\) at equilibrium?
(1). 0.8889
(2). 0.717
(3). 0.00889
(4). 0.0889
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Amonst the given options which of the following
molecules/ion acts as a Lewis acid?
(1). H2O
(2). BF3
(3). OH-
(4). NH3
(1). H2O
(2). BF3
(3). OH-
(4). NH3
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The equilibrium concentrations of the species in the reaction \(A\,+\,B\, \rightleftharpoons\, C \,+\,D\) are 2, 3, 10, and 6 mol L-1, respectively at 300 K. \(\delta G^\circ\) for the reaction is (R = 2 cal/mol K).
(1). -137.26 cal
(2). -1381.80 cal
(3). -13.73 cal
(4). 1372.60 cal
(1). -137.26 cal
(2). -1381.80 cal
(3). -13.73 cal
(4). 1372.60 cal
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The pH of the solution containing 50 mL each of 0.10 M sodium acetate and 0.01 M acetic acid is
[Given aKa, of CH3COOH = 4.57]
(1). 5.57
(2). 3.57
(3). 4.57
(4). 2.57
[Given aKa, of CH3COOH = 4.57]
(1). 5.57
(2). 3.57
(3). 4.57
(4). 2.57
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
What mass of 95% pure CaCO3 will be required to neutralise 50 mL of 0.5 M HCl solution according to the following reaction?

[Calculate upto second place of decimal point]
(1). 1.25 g
(2). 1.32 g
(3). 3.65 g
(4). 9.50 g

[Calculate upto second place of decimal point]
(1). 1.25 g
(2). 1.32 g
(3). 3.65 g
(4). 9.50 g
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
\(\text{3O}_2\,\left(g\right) \rightarrow\, \text{2O}_3 \left(g\right) \)
for the above reaction at 298 K, Kc is found to be \(3.0 \times 10^-{59}\). If the concentration of O2 at equilibrium is 0.040 M then concentration of O2 in M is
(1). \( 4.38 \times 10^{-32}\)
(2). \(1.9 \times 10^{-63}\)
(3). \(2.4 \times 10^{31}\)
(4). \(1.2 \times 10^{21}\)
for the above reaction at 298 K, Kc is found to be \(3.0 \times 10^-{59}\). If the concentration of O2 at equilibrium is 0.040 M then concentration of O2 in M is
(1). \( 4.38 \times 10^{-32}\)
(2). \(1.9 \times 10^{-63}\)
(3). \(2.4 \times 10^{31}\)
(4). \(1.2 \times 10^{21}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The pKb of dimethylamine and pka of acetic acid are 3.27 and 4.77 respectively at T (K). The correct option for the pH of dimethylammonium acetate solution is:
(1). 8.50
(2). 5.50
(3). 7.75
(4). 6.25
(1). 8.50
(2). 5.50
(3). 7.75
(4). 6.25
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which will make basic buffer ?
(1). 100 mL of 0.1 M CH3COOH + 100 mL of 0.1 mL NaOH
(2). 100 mL of 0.1 M HCl + 200 mL of 0.1 m NH4OH
(3). 100 mL of 0.1 M HCl + 100 mL of 0.1 M NaOH
(4). 50 mL of 0.1 period M NaOH elements + 25 mL of 0.1 M CH3COOH
(1). 100 mL of 0.1 M CH3COOH + 100 mL of 0.1 mL NaOH
(2). 100 mL of 0.1 M HCl + 200 mL of 0.1 m NH4OH
(3). 100 mL of 0.1 M HCl + 100 mL of 0.1 M NaOH
(4). 50 mL of 0.1 period M NaOH elements + 25 mL of 0.1 M CH3COOH
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
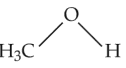
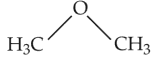
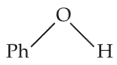
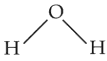
The compound that is most difficult to protonate is
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
For the chemical reaction
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
the correct option is
(1).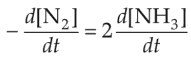
(2).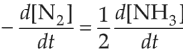
(3).
(4).
N2(g) + 3H2(g) \(\rightleftharpoons\) 2NH3(g)
the correct option is
(1).
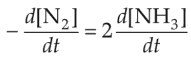
(2).
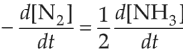
(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
pH of a saturated solution of Ca(OH)2 is 9. The solubility product (ksp) of Ca(OH)2 is
(1). 0.25 x 10-10
(2). 0.125 x 10-15
(3). 0.5 x 10-10
(4). 0.5 x 10-15
(1). 0.25 x 10-10
(2). 0.125 x 10-15
(3). 0.5 x 10-10
(4). 0.5 x 10-15
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Following solutions were prepared by mixing different volumes of NaOH and HCl of different concentrations:
A. \( \text{60 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{40 mL} \frac{\text{M}}{\text{10}}\)NaOH
B. \( \text{55 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{45 mL} \frac{\text{M}}{\text{10}}\)NaOH
C. A. \( \text{75 mL} \frac{\text{M}}{\text{5}}\)HCl + \( \text{25 mL} \frac{\text{M}}{\text{5}}\)NaOH
D. \( \text{100 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{100 mL} \frac{\text{M}}{\text{10}}\)NaOH
pH of which one of them will be equal to 1 ?
(1). C
(2). D
(3). A
(4). B
A. \( \text{60 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{40 mL} \frac{\text{M}}{\text{10}}\)NaOH
B. \( \text{55 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{45 mL} \frac{\text{M}}{\text{10}}\)NaOH
C. A. \( \text{75 mL} \frac{\text{M}}{\text{5}}\)HCl + \( \text{25 mL} \frac{\text{M}}{\text{5}}\)NaOH
D. \( \text{100 mL} \frac{\text{M}}{\text{10}}\)HCl + \( \text{100 mL} \frac{\text{M}}{\text{10}}\)NaOH
pH of which one of them will be equal to 1 ?
(1). C
(2). D
(3). A
(4). B
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The solubility of BaSO4 in water is 2.42 × 10–3gL–1 at 298 K. The value of its solubility product (Ksp) will be
(Given molar mass of BaSO4 = 233 g mol–1)
(1). 1.08 × 10–8 mol2L–2
(2). 1.08 × 10–14 mol2L–2
(3). 1.08 × 10–12 mol2L–2
(4). 1.08 × 10–10 mol2L–2
(Given molar mass of BaSO4 = 233 g mol–1)
(1). 1.08 × 10–8 mol2L–2
(2). 1.08 × 10–14 mol2L–2
(3). 1.08 × 10–12 mol2L–2
(4). 1.08 × 10–10 mol2L–2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the following conditions will favour maximum formation of the product in the reaction,
A2(g) + B2(g) \(\rightleftharpoons \) X2(g) \(\Delta_r\)H = -XkJ?
(1). High temperature and low pressure
(2). High temperature and high pressure
(3). Low temperature and low pressure
(4). Low temperature and high pressure
A2(g) + B2(g) \(\rightleftharpoons \) X2(g) \(\Delta_r\)H = -XkJ?
(1). High temperature and low pressure
(2). High temperature and high pressure
(3). Low temperature and low pressure
(4). Low temperature and high pressure
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The equilibrium constants of the following are:
N2 + 3H2 \(\rightleftharpoons\) 2NH3 ------ K1
N2 + O2 \(\rightleftharpoons\) 2NO ------ K2
H2 + \(\frac{\text{1}}{\text{2}}\)O2 \(\rightarrow \) H2O ------ K3
The equilibrium constant (K) of the reaction:
2NH3 + \(\frac{\text{5}}{\text{2}}\)O2 \(\rightleftharpoons\) 2NO + 3H2O, will be
(1). \(\text{K}_2^3\, \text{K}_3\)/\(\text{K}_1\)
(2). \(\text{K}_1\, \text{K}_3^3\)/\(\text{K}_2\)
(3). \(\text{K}_2\, \text{K}_3^3\)/\(\text{K}_1\)
(4). \(\text{K}_2\, \text{K}_3\)/\(\text{K}_1\)
N2 + 3H2 \(\rightleftharpoons\) 2NH3 ------ K1
N2 + O2 \(\rightleftharpoons\) 2NO ------ K2
H2 + \(\frac{\text{1}}{\text{2}}\)O2 \(\rightarrow \) H2O ------ K3
The equilibrium constant (K) of the reaction:
2NH3 + \(\frac{\text{5}}{\text{2}}\)O2 \(\rightleftharpoons\) 2NO + 3H2O, will be
(1). \(\text{K}_2^3\, \text{K}_3\)/\(\text{K}_1\)
(2). \(\text{K}_1\, \text{K}_3^3\)/\(\text{K}_2\)
(3). \(\text{K}_2\, \text{K}_3^3\)/\(\text{K}_1\)
(4). \(\text{K}_2\, \text{K}_3\)/\(\text{K}_1\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Concentration of the Ag+ ions in a saturated solution of Ag2C2O4 is 2.2 × 10–4mol L–1. Solubility product of Ag2C2O4 is
(1). 5.3 × 10–12
(2). 2.42 × 10–8
(3). 2.66 × 10–12
(4). 4.5 × 10–11
(1). 5.3 × 10–12
(2). 2.42 × 10–8
(3). 2.66 × 10–12
(4). 4.5 × 10–11
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A 20 litre container at of 400 K contains CO2(g) at pressure 0.4 atm and an excess SrO (neglect the volume of solid SrO). The volume of the containers is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of CO2 attains its maximum value, will be
[Given : SrCO3(s) \(\rightleftharpoons\) SrO(s) + CO2 (g).
K = 1.6 atm]
(1). 2 litre
(2). 5 litre
(3). 10 litre
(4). 4 litre
[Given : SrCO3(s) \(\rightleftharpoons\) SrO(s) + CO2 (g).
K = 1.6 atm]
(1). 2 litre
(2). 5 litre
(3). 10 litre
(4). 4 litre
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The product obtained as a result of a reaction of nitrogen with CaC2 is
(1). CaCN
(2). CaCN3
(3). Ca2CN
(4). Ca(CN)2
(1). CaCN
(2). CaCN3
(3). Ca2CN
(4). Ca(CN)2
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Among the following , the correct order of acidity is
(1). HClO < HClO2 < HClO3 < HClO4
(2). HClO2 < HClO < HClO3 < HClO4
(3). HClO4 < HClO2 < HClO < HClO3
(4). HClO3 < HClO4 < HClO2 < HClO
(1). HClO < HClO2 < HClO3 < HClO4
(2). HClO2 < HClO < HClO3 < HClO4
(3). HClO4 < HClO2 < HClO < HClO3
(4). HClO3 < HClO4 < HClO2 < HClO
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following statements is false ?
(1). Ca2+ ions are important in blood clotting
(2). Ca2+ ions are not important in maintaining the regular beating of the heart .
(3). Mg2+ions are important in the green parts of plants
(4). Mg2+ ions form a complex with ATP
(1). Ca2+ ions are important in blood clotting
(2). Ca2+ ions are not important in maintaining the regular beating of the heart .
(3). Mg2+ions are important in the green parts of plants
(4). Mg2+ ions form a complex with ATP
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
