Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 19
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
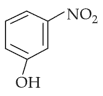
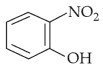
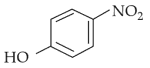
Intramolecular hydrogen bonding is present in
(1).
(2).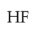
(3).
(4).
(1).

(2).
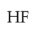
(3).

(4).

Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The number of protons, neutrons and electrons in
\(^{175} _{71}Lu\), respectively are:
(1). 104, 71 and 71
(2). 71,71 and 104
(3). 175, 104 and 71
(4). 71, 104 and 71
(1). 104, 71 and 71
(2). 71,71 and 104
(3). 175, 104 and 71
(4). 71, 104 and 71
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The ratio of the wavelengths of the light absorbed by a Hydrogen atom when it undergoes n = 2 → n = 3 and n = 4 → n = 6 transitions, respectively, is
(1). \(\displaystyle \frac{1}{4}\)
(2). \(\displaystyle \frac{1}{36}\)
(3). \(\displaystyle \frac{1}{16}\)
(4). \(\displaystyle \frac{1}{9}\)
(1). \(\displaystyle \frac{1}{4}\)
(2). \(\displaystyle \frac{1}{36}\)
(3). \(\displaystyle \frac{1}{16}\)
(4). \(\displaystyle \frac{1}{9}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Dalton's Atomic theory could not explain which of the following?
(1). Law of gaseous volume
(2). Law of conservation of mass
(3). Law of constant proportion
(4). Law of multiple proportion
(1). Law of gaseous volume
(2). Law of conservation of mass
(3). Law of constant proportion
(4). Law of multiple proportion
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Energy and radius of first Bohr orbit of He+ and Li2+ are
[Given RH = 2.18 × 10-18 J, ao = 52.9 pm]
(1). \(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(2). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)
(3). \(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 26.4\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(4). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)
[Given RH = 2.18 × 10-18 J, ao = 52.9 pm]
(1). \(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(2). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)
(3). \(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 26.4\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(4). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List-I with List-II
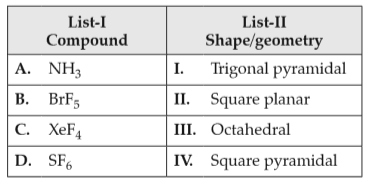
Choose the correct answer from the options given below:
(1). A-III, B-IV, C-I, D-II
(2). A-II, B-III, C-IV, D-I
(3). A-I, B-IV, C-II, D-III
(4). A-II, B-IV, C-III, D-I

Choose the correct answer from the options given below:
(1). A-III, B-IV, C-I, D-II
(2). A-II, B-III, C-IV, D-I
(3). A-I, B-IV, C-II, D-III
(4). A-II, B-IV, C-III, D-I
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements:
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
(1). Statement I is true, but Statement II is false.
(2). Statement I is false, but Statement II is true..
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
(1). Statement I is true, but Statement II is false.
(2). Statement I is false, but Statement II is true..
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The energy of an electron in the ground state (n = 1) for \(\text{He}^+\) ion is \(-x\text{J}\), then that for an electron in n = 2 state for \(\text{Be}^{3+}\) ion in J is:
(1). \(\displaystyle-\,4x\)
(2). \(\displaystyle-\,\frac{4}{9}x\)
(3). \(\displaystyle-\,x\)
(4). \(\displaystyle-\,\frac{x}{9}\)
(1). \(\displaystyle-\,4x\)
(2). \(\displaystyle-\,\frac{4}{9}x\)
(3). \(\displaystyle-\,x\)
(4). \(\displaystyle-\,\frac{x}{9}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Match List-I with List-II
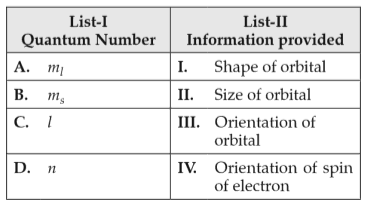
Choose the correct answer from the options given below:
(1). A-III, B-IV, C-II, D-I
(2). A-II, B-I, C-IV, D-III
(3). A-I, B-III, C-II, D-IV
(4). A-III, B-IV, C-I, D-II

Choose the correct answer from the options given below:
(1). A-III, B-IV, C-II, D-I
(2). A-II, B-I, C-IV, D-III
(3). A-I, B-III, C-II, D-IV
(4). A-III, B-IV, C-I, D-II
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Select the correct Statements from the following:
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
(1). C, D and E only
(2). A and E only
(3). B, C and E only
(4). A, B and C only
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 x 10 kg.
C. All the isotopes of a given elements show sme chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton's atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below:
(1). C, D and E only
(2). A and E only
(3). B, C and E only
(4). A, B and C only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The relation between nm, (nm = the number of permissible values of magentic quantum number (m)) for a given value of aziuthal quantum number (l), is
(1). \(l\,=\,2n_m\,+\,1\)
(2). \(n_m\,=\,2l^2\,+\,1\)
(3). \(n_m\,=\,l\,+\,2\)
(4). \(l\,=\,\frac{n_m\,-\,1}{2}\)
(1). \(l\,=\,2n_m\,+\,1\)
(2). \(n_m\,=\,2l^2\,+\,1\)
(3). \(n_m\,=\,l\,+\,2\)
(4). \(l\,=\,\frac{n_m\,-\,1}{2}\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Identify the incorrect statement from thefollowing:
(1). All the five 5d orbitals are different in size when compared to the respective 4d orbitals.
(2). All the five 4d orbitals have shapes similar tothe respective 3d orbitals.
(3). In an atom, all the five 3d orbitals are equal in energy in free state.
(4). The shapes of dxy, dyz and dzx orbitals are similar to each other; and dx2-y2and dz2 are similar to each other.
(1). All the five 5d orbitals are different in size when compared to the respective 4d orbitals.
(2). All the five 4d orbitals have shapes similar tothe respective 3d orbitals.
(3). In an atom, all the five 3d orbitals are equal in energy in free state.
(4). The shapes of dxy, dyz and dzx orbitals are similar to each other; and dx2-y2and dz2 are similar to each other.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
If radius of second Bohr orbit of the He+ ion is 105.8 pm, what is the radius of third Bohr orbit of Li2+ ion?
(1). 158.7 pm
(2). 15.87 pm
(3). 1.587 pm
(4). 158.7 Ả
(1). 158.7 pm
(2). 15.87 pm
(3). 1.587 pm
(4). 158.7 Ả
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A particular station of All India Radio, New Delhi, broadcasts on a frequency of 1,368 kHz (kilohertz). The wavelength of the electromagnetic radiation emitted by the transmitter is : [speed of light c=3.0 × 108 ms-1]
(1). 219.3 m
(2). 219.2 m
(3). 2192 m
(4). 21.92 cm
(1). 219.3 m
(2). 219.2 m
(3). 2192 m
(4). 21.92 cm
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
4d, 5p, 5f, and 6p orbitals are arranged in the order of decreasing energy. The correct option is
(1). 6p > 5f > 5p > 4d
(2). 6p > 5f > 4d > 5p
(3). 5f > 6p > 4d > 5p
(4). 5f > 6p > 5p > 4d
(1). 6p > 5f > 5p > 4d
(2). 6p > 5f > 4d > 5p
(3). 5f > 6p > 4d > 5p
(4). 5f > 6p > 5p > 4d
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which of the following series of transitions in the spectrum of hydrogen atom falls in visible region ?
(1). Balmer series
(2). Paschen series
(3). Brackett series
(4). Lyman series
(1). Balmer series
(2). Paschen series
(3). Brackett series
(4). Lyman series
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one is a wrong statement ?
(1). The value of m for dz2 is zero.
(2). The electronic configuration of N atom is

(3). An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers.
(4). Total orbital angular momentum of electron in 's' orbital is equal to zero.
(1). The value of m for dz2 is zero.
(2). The electronic configuration of N atom is

(3). An orbital is designated by three quantum numbers while an electron in an atom is designated by four quantum numbers.
(4). Total orbital angular momentum of electron in 's' orbital is equal to zero.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one is the wrong statement ?
(1). The energy of 2s orbital is less than the energy of 2p orbital in case of Hydrogen like atoms.
(2). de-Broglie's wavelength is given by \(\lambda\,=\,\frac{\text{h}}{\text{mv}}\)
where m = mass of the particle, v = group velocity of the particle.
(3). The uncertainty principle is \(\Delta \text{E} \times \Delta \text{t}\) ≥ \(\frac{\text{h}}{\text{4} \pi\)
(4). Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement.
(1). The energy of 2s orbital is less than the energy of 2p orbital in case of Hydrogen like atoms.
(2). de-Broglie's wavelength is given by \(\lambda\,=\,\frac{\text{h}}{\text{mv}}\)
where m = mass of the particle, v = group velocity of the particle.
(3). The uncertainty principle is \(\Delta \text{E} \times \Delta \text{t}\) ≥ \(\frac{\text{h}}{\text{4} \pi\)
(4). Half-filled and fully filled orbitals have greater stability due to greater exchange energy, greater symmetry and more balanced arrangement.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Two electrons occupying the same orbital are distinguished by
(1). magnetic quantum number.
(2). azimuthal quantum number.
(3). spin quantum number.
(4). principal quantum number.
(1). magnetic quantum number.
(2). azimuthal quantum number.
(3). spin quantum number.
(4). principal quantum number.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
