Home >> My Performance >> My Topic Test Performance >> My Question Performance
My Question Performance Summary in Full Tests !
Questions Available: 15
Questions Attempted: 10
Number of Attempts: 15
Correct Attempts: 8
Total Time Spent: 00:30
Avg Time Per Question: 00:02
My Question Performance Summary in Full Tests
Which one is not correct mathematical equationfor Dalton's Law of partial pressure?
Here p = total pressure of gaseous mixture
(1). \(p\,=\,p_1\,+\,p_2\,+\,p_3\)
(2). \(p\,=\,n_1 \frac{RT}{V}\,+\,n_2 \frac{RT}{V}\,+\,n_3 \frac{RT}{V}\)
(3). \(p_i\,=\, x_ip_i\) where \(p_i\) = partial pressure of \(i^{th}\) gas and \(x_i\) = mole fraction of \(i^th\) gas in gaseous mixture
(4). \(p_i\,=\, x_ip_i^\circ\) where \(p_i^\circ\) = partial pressure of \(i^{th}\) gas and \(x_i\) = mole fraction of \(i^th\) gas in gaseous mixture
Here p = total pressure of gaseous mixture
(1). \(p\,=\,p_1\,+\,p_2\,+\,p_3\)
(2). \(p\,=\,n_1 \frac{RT}{V}\,+\,n_2 \frac{RT}{V}\,+\,n_3 \frac{RT}{V}\)
(3). \(p_i\,=\, x_ip_i\) where \(p_i\) = partial pressure of \(i^{th}\) gas and \(x_i\) = mole fraction of \(i^th\) gas in gaseous mixture
(4). \(p_i\,=\, x_ip_i^\circ\) where \(p_i^\circ\) = partial pressure of \(i^{th}\) gas and \(x_i\) = mole fraction of \(i^th\) gas in gaseous mixture
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : In a particular point defect, anionic solid is electrically neutral, even if few of its cations are missing from its unit cells.
Reason (R) : In an ionic solid, Frenkel defectarises due to dislocation of cation from its latticesite to interstitial site, maintaining overall electrical neutrality.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Both (A) and (R) are correct and (R) is the correct explanation of (A)
(2). Both (A) and (R) are correct but (R) is not the correct explanation of (A)
(3). (A) is correct but (R) is not correct
(4). (A) is not correct but (R) is correct
Assertion (A) : In a particular point defect, anionic solid is electrically neutral, even if few of its cations are missing from its unit cells.
Reason (R) : In an ionic solid, Frenkel defectarises due to dislocation of cation from its latticesite to interstitial site, maintaining overall electrical neutrality.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Both (A) and (R) are correct and (R) is the correct explanation of (A)
(2). Both (A) and (R) are correct but (R) is not the correct explanation of (A)
(3). (A) is correct but (R) is not correct
(4). (A) is not correct but (R) is correct
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Among the following, choose the ones with equal number of atoms.
A. 212 g of \(Na_2 CO_3 \left(s\right)\) [molar mass = 106 g]
B. 248 g of\( Na_2 O \left(s\right)\) [molar mass = 62 g]
C. 240 g of \(NaOH \left(s\right)\) [molar mass = 40 g]
D. 12 g of \(H_2 \left(g\right)\) [molar mass = 2 g]
E. 220 g of \(CO_2 \left(g \right)\) [molar mass = 44 g]
Choose the correct answer from the options given below :
(1). B, D, and E only
(2). A, B, and C only
(3). A, B, and D only
(4). B, C, and D only
A. 212 g of \(Na_2 CO_3 \left(s\right)\) [molar mass = 106 g]
B. 248 g of\( Na_2 O \left(s\right)\) [molar mass = 62 g]
C. 240 g of \(NaOH \left(s\right)\) [molar mass = 40 g]
D. 12 g of \(H_2 \left(g\right)\) [molar mass = 2 g]
E. 220 g of \(CO_2 \left(g \right)\) [molar mass = 44 g]
Choose the correct answer from the options given below :
(1). B, D, and E only
(2). A, B, and C only
(3). A, B, and D only
(4). B, C, and D only
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A compound X contains 32 % of A, 20 % of B2 and remaining percentage of C. Then, the empirical formula of X is:
(1). \(\text{AB}_2\text{C}_2\)
(2). \(\text{ABC}_4\)
(3). \(\text{A}_2\text{BC}_2\)
(4). \(\text{ABC}_3\)
(1). \(\text{AB}_2\text{C}_2\)
(2). \(\text{ABC}_4\)
(3). \(\text{A}_2\text{BC}_2\)
(4). \(\text{ABC}_3\)
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The highest number of helium atoms is in
(1). 4 g of helium
(2). 2.271098 L of helium at STP
(3). 4 mol of helium
(4). 4 u of helium
(1). 4 g of helium
(2). 2.271098 L of helium at STP
(3). 4 mol of helium
(4). 4 u of helium
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
1 gram of sodium hydroxide was treated with 25mL of 0.75 M HCl solution, the mass of sodium hydroxide left unreacted is equal to
(1). Zero mg
(2). 200 mg
(3). 750 mg
(4). 250 mg
(1). Zero mg
(2). 200 mg
(3). 750 mg
(4). 250 mg
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Intermolecular forces are forces of attraction andrepulsion between interacting particles that willinclude:
A. dipole - dipole forces.
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from theoptions given below:
(1). A, B, C, D are correct
(2). A, B, C, E are correct
(3). A, C, D, E are correct
(4). B, C, D, E are correct
A. dipole - dipole forces.
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from theoptions given below:
(1). A, B, C, D are correct
(2). A, B, C, E are correct
(3). A, C, D, E are correct
(4). B, C, D, E are correct
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The right option for the mass of CO2 produced by heating 20g of 20% pure limestone is
(Atomic mass of Ca = 40)
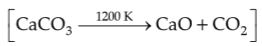
(1). 1.76 g
(2). 2.64 g
(3). 1.32 g
(4). 1.12 g

(1). 1.76 g
(2). 2.64 g
(3). 1.32 g
(4). 1.12 g
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Choose the correct statement:
(1). Diamond and graphite have two dimensional network.
(2). Diamond is covalent and graphite is ionic.
(3). Diamond is sp3 hybridized and graphite is sp2 hybridized.
(4). Both diamond and graphite are used as dry lubricants.
(1). Diamond and graphite have two dimensional network.
(2). Diamond is covalent and graphite is ionic.
(3). Diamond is sp3 hybridized and graphite is sp2 hybridized.
(4). Both diamond and graphite are used as dry lubricants.
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In one molal solution that contains 0.5 mole of asolute, there is
(1). 500 mL of solvent
(2). 500 g of solvent
(3). 100 mL of solvent
(4). 1000 g of solvent
(1). 500 mL of solvent
(2). 500 g of solvent
(3). 100 mL of solvent
(4). 1000 g of solvent
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
A 10.0 L flask contains 64g ofoxygen at \(\text{27}^\circ C\). (Assume O2 gas is behaving ideally). The pressure inside theflask in bar is (Given R = 0.0831 L bar K-1 mol-1)
(1). 2.5
(2). 498.6
(3). 49.8
(4). 4.9
(1). 2.5
(2). 498.6
(3). 49.8
(4). 4.9
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Copper crystallises in fcc unit cell with cell edgelength of \(3.608 \times 10^8\) cm. The density of copper is 8.92 g cm-3. Calculate the atomic mass of copper.
(1). 63.1u
(2). 31.55 u
(3). 60 u
(4). 65 u
(1). 63.1u
(2). 31.55 u
(3). 60 u
(4). 65 u
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
Which one of the followings has maximum number
of atoms ?
(1). 1g of Mg(s) [Atomic mass of Mg = 24]
(2). 1g of O2 (g) [Atomic mass of O = 16]
(3). 1g of Li(s) [Atomic mass of Li = 7]
(4). 1g of Ag(s) [Atomic mass of Ag = 108]
(1). 1g of Mg(s) [Atomic mass of Mg = 24]
(2). 1g of O2 (g) [Atomic mass of O = 16]
(3). 1g of Li(s) [Atomic mass of Li = 7]
(4). 1g of Ag(s) [Atomic mass of Ag = 108]
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
The number of moles of hydrogen molecules required to produce 20 moles of ammonia through Haber's process is
(1). 20
(2). 30
(3). 40
(4). 10
(1). 20
(2). 30
(3). 40
(4). 10
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
In which case is number of molecules of water maximum ?
(1). 10–3 mol of water
(2). 0.00224 L of water vapours at 1 atm and 273 K r
(3). 0.18 g of water
(4). 18 mL of wate
(1). 10–3 mol of water
(2). 0.00224 L of water vapours at 1 atm and 273 K r
(3). 0.18 g of water
(4). 18 mL of wate
Number of Attempts: 2
Correct Attempts: 1
Time Taken: 00:04
Average Time: 00:02
