Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 64
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
An element has a body centered cubic (bcc)
structure with a cell edge of 288 pm. The atomic
radius is:
(1). \(\frac{\sqrt{2}}{4} \times 288\) pm
(2). \(\frac{4}{\sqrt{3}} \times 288\) pm
(3). \(\frac{4}{\sqrt{2}} \times 288\) pm
(4). \(\frac{\sqrt{3}}{4} \times 288\) pm
(1). \(\frac{\sqrt{2}}{4} \times 288\) pm
(2). \(\frac{4}{\sqrt{3}} \times 288\) pm
(3). \(\frac{4}{\sqrt{2}} \times 288\) pm
(4). \(\frac{\sqrt{3}}{4} \times 288\) pm
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
A gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions. The correct option about the gas and its compressibility factor (Z) is
(1). Z > 1 and repulsive forces and dominant
(2). Z < 1 and attractive forces and dominant
(3). Z < 1 and repulsive forces and dominant
(4). Z > 1 and attractive forces and dominant
(1). Z > 1 and repulsive forces and dominant
(2). Z < 1 and attractive forces and dominant
(3). Z < 1 and repulsive forces and dominant
(4). Z > 1 and attractive forces and dominant
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The biodegradable polymer is
(1). nylon-2-nylon 6
(2). nylon-6
(3). Buna-S
(4). nylon-6, 6
(1). nylon-2-nylon 6
(2). nylon-6
(3). Buna-S
(4). nylon-6, 6
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which oxide of nitrogen is not a common pollutant introduced into the atmosphere both due to natural and human activity ?
(1). NO
(2). N2O
(3). NO2
(4). N2O5
(1). NO
(2). N2O
(3). NO2
(4). N2O5
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given van der Waals constant for NH3, H2, O2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied ?
(1). CO2
(2). O2
(3). H2
(4). NH3
(1). CO2
(2). O2
(3). H2
(4). NH3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match items of Column I with the items of Column II and assign the correct code.
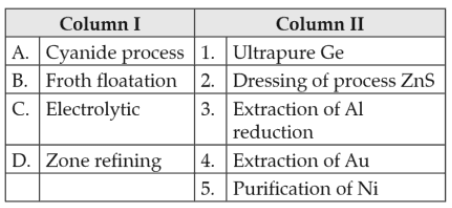
Codes
(1). A - 2, B - 3, C - 1, D - 5
(2). A - 1, B - 2, C - 3, D - 4
(3). A - 3, B - 4, C - 5, D - 1
(4). A - 4, B - 2, C - 3, D - 1

Codes
(1). A - 2, B - 3, C - 1, D - 5
(2). A - 1, B - 2, C - 3, D - 4
(3). A - 3, B - 4, C - 5, D - 1
(4). A - 4, B - 2, C - 3, D - 1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Fog is a colloidal solution of
(1). Gas in liquid
(2). Solid in gas
(3). Gas in gas
(4). Liquid in gas
(1). Gas in liquid
(2). Solid in gas
(3). Gas in gas
(4). Liquid in gas
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statements about hydrogen is incorrect ?
(1). Hydrogen never acts as cation in ionic salts
(2). Hydronium ion , H3O+ exists freely in solution
(3). Dihydrogen does not act as a reducing agent
(4). Hydrogen has three isotopes of which tritium is the most common
(1). Hydrogen never acts as cation in ionic salts
(2). Hydronium ion , H3O+ exists freely in solution
(3). Dihydrogen does not act as a reducing agent
(4). Hydrogen has three isotopes of which tritium is the most common
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one is an example of heterogeneous catalysis?
(1). Hydrolysis of sugar catalysed by H+ ions.
(2). Decomposition of ozone in presence of nitrogen monoxide.
(3). Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron.
(4). Oxidation of sulphur dioxide into sulphur trioxide in the presence of oxides of nitrogen.
(1). Hydrolysis of sugar catalysed by H+ ions.
(2). Decomposition of ozone in presence of nitrogen monoxide.
(3). Combination between dinitrogen and dihydrogen to form ammonia in the presence of finely divided iron.
(4). Oxidation of sulphur dioxide into sulphur trioxide in the presence of oxides of nitrogen.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
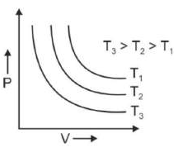
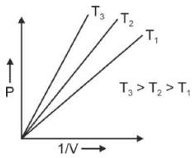
Which amonst the following options is correct graphical representation of Boyle's law?
(1).
(2).
(3).
(4).
(1).

(2).

(3).

(4).

Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
A compound is formed by two elements A and B.The elements B forms cubic close packed structure and atoms of A occupy \(\frac{1}{3}\) of tetrahedralvoids. If the formula of the compound is AxBy, thenthe value of x + y is in option.
(1). 4
(2). 3
(3). 2
(4). 5
(1). 4
(2). 3
(3). 2
(4). 5
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Some tranquilizers are listed below. Which onefrom the following belongs to barbiturates?
(1). Meprobamate
(2). Valium
(3). Veronal
(4). Chlordiazepoxide
(1). Meprobamate
(2). Valium
(3). Veronal
(4). Chlordiazepoxide
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statements are NOTcorrect?
A. Hydrogen is used to reduce heavy metal oxidesto metals.
B. Heavy water is used to study reactionmechanism.
C. Hydrogen is used to make saturated fats formoils.
D. The H-H bond dissociation enthalpy is lowestas compared to a single bond between twoatoms of any element.
E. Hydrogen reduces oxides of metals that aremore active than iron.
Choose the most approriate answer from theoptions given below:
(1). B, D only
(2). D, E only
(3). A, B, C only
(4). B, C, D, E only
A. Hydrogen is used to reduce heavy metal oxidesto metals.
B. Heavy water is used to study reactionmechanism.
C. Hydrogen is used to make saturated fats formoils.
D. The H-H bond dissociation enthalpy is lowestas compared to a single bond between twoatoms of any element.
E. Hydrogen reduces oxides of metals that aremore active than iron.
Choose the most approriate answer from theoptions given below:
(1). B, D only
(2). D, E only
(3). A, B, C only
(4). B, C, D, E only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following statements is correct?
(1). All enzymes that utilise ATP in phosphate transfer require Ca as the cofactor.
(2). The bone in human body is an inert and unchanging substance.
(3). Mg plays roles in neuromuscular function and interneuronal transmission.
(4). The daily requirement of Mg and Ca in the human body is estimated to be 0.2 - 0.3 g.
(1). All enzymes that utilise ATP in phosphate transfer require Ca as the cofactor.
(2). The bone in human body is an inert and unchanging substance.
(3). Mg plays roles in neuromuscular function and interneuronal transmission.
(4). The daily requirement of Mg and Ca in the human body is estimated to be 0.2 - 0.3 g.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements :
Statement I: The nutrient deficient water bodies lead to eutrophication.
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose thecorrect answer from the options given below:
(1). Both Statement I and Statement II are false.
(2). StatementI is true but Statement II isfalse.
(3). Statement I is false but Statement II istrue.
(4). Both Statement I and Statement II are true.
Statement I: The nutrient deficient water bodies lead to eutrophication.
Statement II : Eutrophication leads to decrease in the level of oxygen in the water bodies.
In the light of the above statements, choose thecorrect answer from the options given below:
(1). Both Statement I and Statement II are false.
(2). StatementI is true but Statement II isfalse.
(3). Statement I is false but Statement II istrue.
(4). Both Statement I and Statement II are true.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Pumice stone is an example of-
(1). gel
(2). solid sol
(3). foam
(4). sol
(1). gel
(2). solid sol
(3). foam
(4). sol
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The reaction that does NOT take place in blastfurnace between 900 K to 1500 K temperaturerange during extraction of iron is:
(1). FeO + CO → Fe + CO2
(2). C + CO2 → 2CO
(3). CaO + SiO2 → CaSiO3
(4). Fe2O3 + CO → 2FeO + CO2
(1). FeO + CO → Fe + CO2
(2). C + CO2 → 2CO
(3). CaO + SiO2 → CaSiO3
(4). Fe2O3 + CO → 2FeO + CO2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
What fraction of one edge centred octahedral voidlies in one unit cell of fcc?
(1). \(\frac{1}{3}\)
(2). \(\frac{1}{4}\)
(3). \(\frac{1}{12}\)
(4). \(\frac{1}{2}\)
(1). \(\frac{1}{3}\)
(2). \(\frac{1}{4}\)
(3). \(\frac{1}{12}\)
(4). \(\frac{1}{2}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements:
Statement I: In the coagulation of a negativesol, the flocculating power of the three given ionsis in the order
AI3+ > Ba2+ > Na+
Statement II : In the coagulation of a positivesol, the flocculating power of the three given saltsis in the orderNaCl > Na2SO4 > Na3PO3
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but statement II is correct.
Statement I: In the coagulation of a negativesol, the flocculating power of the three given ionsis in the order
AI3+ > Ba2+ > Na+
Statement II : In the coagulation of a positivesol, the flocculating power of the three given saltsis in the orderNaCl > Na2SO4 > Na3PO3
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but statement II is correct.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which statement regarding polymers isnot correct?
(1). Elastomers have polymer chains held togetherby weak intermolecular forces
(2). Fibers possess high tensile strength
(3). Thermoplastic polymers are capable of repeatedly softening and hardening on heating and cooling respectively
(4). Thermosetting polymers are reusable
(1). Elastomers have polymer chains held togetherby weak intermolecular forces
(2). Fibers possess high tensile strength
(3). Thermoplastic polymers are capable of repeatedly softening and hardening on heating and cooling respectively
(4). Thermosetting polymers are reusable
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The incorrect statement regarding enzymes is
(1). Enzymes are biocatalysts.
(2). Like chemical catalysts enzymes reduce theactivation energy of bio processes.
(3). Enzymes are polysaccharides.
(4). Enzymes are very specific for a particular reaction and substrate.
(1). Enzymes are biocatalysts.
(2). Like chemical catalysts enzymes reduce theactivation energy of bio processes.
(3). Enzymes are polysaccharides.
(4). Enzymes are very specific for a particular reaction and substrate.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II.
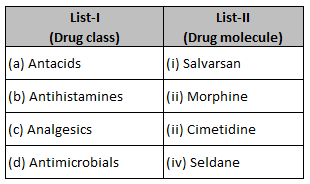
Choose the correct answer from the options givenbelow :
(1). (a) - (iii), (b) - (ii), (c)-(iv), (d) -(i)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d)- (i)
(3). (a) - (i), (b) - (iv), (c)-(ii), (d) – (iii)
(4). (a)-(iv), (b)-– (iii), (c) -(i), (d) - (ii)

Choose the correct answer from the options givenbelow :
(1). (a) - (iii), (b) - (ii), (c)-(iv), (d) -(i)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d)- (i)
(3). (a) - (i), (b) - (iv), (c)-(ii), (d) – (iii)
(4). (a)-(iv), (b)-– (iii), (c) -(i), (d) - (ii)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II.
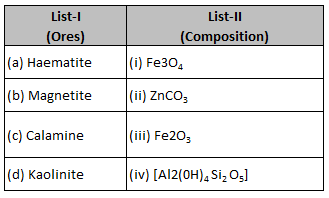
Choose the correct answer from the options givenbelow:
(1). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
(2). (a) - (iii), (b) - (i), (c) - (ii), (d) - (iv)
(3). (a) - (iii), (b) - (i), (c) - (iv), (d) - (ii)
(4). (a) - (i), (b) - (iii), (c) -(ii), (d) - (iv)

Choose the correct answer from the options givenbelow:
(1). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
(2). (a) - (iii), (b) - (i), (c) - (ii), (d) - (iv)
(3). (a) - (iii), (b) - (i), (c) - (iv), (d) - (ii)
(4). (a) - (i), (b) - (iii), (c) -(ii), (d) - (iv)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The pollution due to oxides of sulphur getsenhanced due to the presence of:
(a) particulate matter
(b) Ozone
(c) hydrocarbons
(d) hydrogen peroxide
Choose the most appropriate answer from theoptions given below:
(1). (a), (d) only
(2). (a), (b), (d) only
(3). (b), (c), (d) only
(4). (a), (c), (d) only
(a) particulate matter
(b) Ozone
(c) hydrocarbons
(d) hydrogen peroxide
Choose the most appropriate answer from theoptions given below:
(1). (a), (d) only
(2). (a), (b), (d) only
(3). (b), (c), (d) only
(4). (a), (c), (d) only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements:
Statement I : Aspirin and Paracetamol belong to the class of narcotic analgesics.
Statement II : Morphine and Heroin are non-narcotic analgesine In the light of the above statements, choose the correct answer from the options given below
(1). Both Statement I and Statement II are true
(2). Both Statement I and Statement II are false
(3). Statement I is correct but Statement II is false.
(4). Statement I is incorrect but Statement II is true.
Statement I : Aspirin and Paracetamol belong to the class of narcotic analgesics.
Statement II : Morphine and Heroin are non-narcotic analgesine In the light of the above statements, choose the correct answer from the options given below
(1). Both Statement I and Statement II are true
(2). Both Statement I and Statement II are false
(3). Statement I is correct but Statement II is false.
(4). Statement I is incorrect but Statement II is true.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The right option for the statement "Tyndall effect is exhibited by", is:
(1). NaCl solution
(2). Glucose solution
(3). Starch solution
(4). Urea solution
(1). NaCl solution
(2). Glucose solution
(3). Starch solution
(4). Urea solution
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following polymers is prepared by addition polymerisation?
(1). Teflon
(2). Nylon-66
(3). Novolac
(4). Dacron
(1). Teflon
(2). Nylon-66
(3). Novolac
(4). Dacron
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following methods can be used to obtain highly pure metal which is liquid at room
temperature?
(1). Electrolysis
(2). Chromatography
(3). Distillation
(4). Zone refining
(1). Electrolysis
(2). Chromatography
(3). Distillation
(4). Zone refining
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Tritium, a radioactive isotope of hydrogen, emitswhich of the following particles ?
(1). Beta \( \beta^-\)
(2). Alpha \(\alpha\)
(3). Gamma \(\gamma)
(4). Neutron \(\text{n}\)
(1). Beta \( \beta^-\)
(2). Alpha \(\alpha\)
(3). Gamma \(\gamma)
(4). Neutron \(\text{n}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Right option for the number of tetrahedral and octahedral voids in hexagonal primitive unit cell are:
(1). 8, 4
(2). 6, 12
(3). 2, 1
(4). 12, 6
(1). 8, 4
(2). 6, 12
(3). 2, 1
(4). 12, 6
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Among the following alkaline earth metal halides, one which is covalent and soluble in organic solvents is:
(1). Calcium chloride
(2). Strontium chloride
(3). Magnesium chloride
(4). Beryllium chloride
(1). Calcium chloride
(2). Strontium chloride
(3). Magnesium chloride
(4). Beryllium chloride
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The maximum temperature that can be achieved in blast furnace is:
(1). upto 1200 K
(2). upto 2200 K
(3). upto 1900 K
(4). upto 5000 K
(1). upto 1200 K
(2). upto 2200 K
(3). upto 1900 K
(4). upto 5000 K
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correct option for the number of body centred unit cells in all 14 types of Bravais lattice unit cells is:
(1). 7
(2). 5
(3). 2
(4). 3
(1). 7
(2). 5
(3). 2
(4). 3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Choose the correct option for the total pressure (in atm.) in a mixture of 4 g O2 and 2 g H2 confined in a total volume of one litre at 0°C is:
[Given R=0.082 L atm mol-1K-1, T= 273K]
(1). 2.518
(2). 2.602
(3). 25.18
(4). 26.02
[Given R=0.082 L atm mol-1K-1, T= 273K]
(1). 2.518
(2). 2.602
(3). 25.18
(4). 26.02
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II
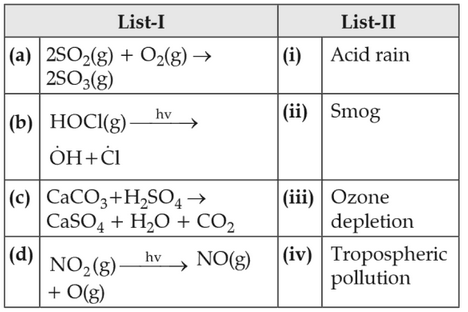
Choose the correct answer from the options given below.
(1). (a)-(i), (b)-(ii), (c)-(iii), (d)-(iv)
(2). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(3). (a)-(iv), (b)-(iii), (c)-(i), (d)-(ii)
(4). (a)-(iii), (b)-(ii), (c)-(iv), (d)-(i)

Choose the correct answer from the options given below.
(1). (a)-(i), (b)-(ii), (c)-(iii), (d)-(iv)
(2). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(3). (a)-(iv), (b)-(iii), (c)-(i), (d)-(ii)
(4). (a)-(iii), (b)-(ii), (c)-(iv), (d)-(i)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
An increase in the concentration of the reactants
of a reaction leads to change in:
(1). heat of reaction
(2). threshold energy
(3). collision frequency
(4). activation energy
(1). heat of reaction
(2). threshold energy
(3). collision frequency
(4). activation energy
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is a natural polymer?
(1). poly (Butadiene-styrene)
(2). polybutadiene
(3). poly (Butadiene-acrylonitrile)
(4). cis- 1, 4-polyisoprene
(1). poly (Butadiene-styrene)
(2). polybutadiene
(3). poly (Butadiene-acrylonitrile)
(4). cis- 1, 4-polyisoprene
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
A mixture of N2 and Ar gases in a cylinder contains 7g of N2 and 8g of Ar. If the total pressure of the mixture of the gases in cylinder is 27 bar, the partial pressure of N2 is:
[Use atomic masses (in gmol-1): N = 14, Ar = 40]
(1). 12 bar
(2). 15 bar
(3). 18 bar
(4). 9 bar
[Use atomic masses (in gmol-1): N = 14, Ar = 40]
(1). 12 bar
(2). 15 bar
(3). 18 bar
(4). 9 bar
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match the following and identify the correct option.
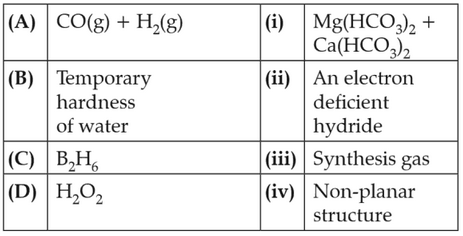
(1). (a) - (iii), (b) - (ii), (c) - (i), (d) - (iv)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d) - (i)
(3). (a) - (i), (b) - (iii), (c) - (ii), (d) - (iv)
(4). (a) - (iii), (b) - (i), (c) - (ii), (d) - (iv)

(1). (a) - (iii), (b) - (ii), (c) - (i), (d) - (iv)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d) - (i)
(3). (a) - (i), (b) - (iii), (c) - (ii), (d) - (iv)
(4). (a) - (iii), (b) - (i), (c) - (ii), (d) - (iv)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
ldentify the correct statements from the folowing:
(1). Blister copper has blistered appearance due to evolution of CO2
(2). Vapour phase refining is carried out for Nickel by Van Arkel method.
(3). Pig iron can be moulded into a variety of shapes.
(4). Wrought iron is impure iron with 4% carbon.
(1). Blister copper has blistered appearance due to evolution of CO2
(2). Vapour phase refining is carried out for Nickel by Van Arkel method.
(3). Pig iron can be moulded into a variety of shapes.
(4). Wrought iron is impure iron with 4% carbon.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Measuring Zeta potential is useful in determining
which property of colloidal solution ?
(1). Solubility
(2). Stability of the colloidal particles
(3). Size of the colloidal particles
(4). Viscosity
(1). Solubility
(2). Stability of the colloidal particles
(3). Size of the colloidal particles
(4). Viscosity
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is a cationic detergent ?
(1). Sodium stearate
(2). Cetyltrimethyl ammonium bromide
(3). Sodium dodecyltbenzene sulphonate
(4). Sodium lauryl sulphate
(1). Sodium stearate
(2). Cetyltrimethyl ammonium bromide
(3). Sodium dodecyltbenzene sulphonate
(4). Sodium lauryl sulphate
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is not correct about carbon monoxide ?
(1). It reduces oxygen carrying ability of blood.
(2). The carboxyhaemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin.
(3). It is produced due to incomplete combustion.
(4). It forms carboxyhaemoglobin.
(1). It reduces oxygen carrying ability of blood.
(2). The carboxyhaemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin.
(3). It is produced due to incomplete combustion.
(4). It forms carboxyhaemoglobin.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Among the following, the one that is not green house gas is
(1). methane.
(2). ozone.
(3). sulphur dioxide.
(4). nitrous oxide.
(1). methane.
(2). ozone.
(3). sulphur dioxide.
(4). nitrous oxide.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one is malachite from the following ?
(1). Cu(OH)2
(2). Fe3O4
(3). CuCO3.Cu(OH)2
(4). CuFeS2
(1). Cu(OH)2
(2). Fe3O4
(3). CuCO3.Cu(OH)2
(4). CuFeS2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is an amphoteric hydroxide ?
(1). Ca(OH)2
(2). Mg(OH)2
(3). Be(OH)2
(4). Sr(OH)2
(1). Ca(OH)2
(2). Mg(OH)2
(3). Be(OH)2
(4). Sr(OH)2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
A compound is formed by cation C and anion A. The anions form hexagonal close packed (hcp) lattice and the cations occupy 75% of octahedral voids. The formula of the compound is
(1). C3A2
(2). C3A4
(3). C4A3
(4). C2A3
(1). C3A2
(2). C3A4
(3). C4A3
(4). C2A3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The method used to remove temporary hardness of water is
(1). Clark’s method.
(2). Ion-exchange method.
(3). Synthetic resins method.
(4). Calgon's method.
(1). Clark’s method.
(2). Ion-exchange method.
(3). Synthetic resins method.
(4). Calgon's method.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which mixture of the solutions will lead to the formation of negatively charged colloidal [AgI]I– sol. ?
(1). 50 mL of 1 M AgNO3 + 50 mL of 2 M KI
(2). 50 mL of 2 M AgNO3 + 50 mL of 1.5 M KI
(3). 50 mL of 0.1 M AgNO3 + 50 mL of 0.1 M I
(4). 50 mL of 1 M AgNO3 + 50 mL of 0.5 M KI
(1). 50 mL of 1 M AgNO3 + 50 mL of 2 M KI
(2). 50 mL of 2 M AgNO3 + 50 mL of 1.5 M KI
(3). 50 mL of 0.1 M AgNO3 + 50 mL of 0.1 M I
(4). 50 mL of 1 M AgNO3 + 50 mL of 0.5 M KI
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match the following
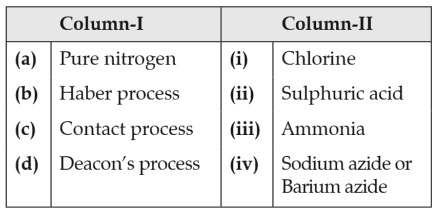
Which of the following is the correct option?
(1). (a) - (ii), (b) - (iv), (c) - (i), (d) - (iii)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d) - (i)
(3). (a) - (iv), (b) - (iii), (c) - (ii), (d) - (i)
(4). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)

Which of the following is the correct option?
(1). (a) - (ii), (b) - (iv), (c) - (i), (d) - (iii)
(2). (a) - (iii), (b) - (iv), (c) - (ii), (d) - (i)
(3). (a) - (iv), (b) - (iii), (c) - (ii), (d) - (i)
(4). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Among the following, the narrow spectrum antibiotic is
(1). ampicillin
(2). amoxycillin
(3). chloramphenicol
(4). penicillin G
(1). ampicillin
(2). amoxycillin
(3). chloramphenicol
(4). penicillin G
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Regarding cross-linked or network polymers, which of the following statements is incorrect ?
(1). They contain strong covalents bonds in their polymer chains.
(2). Examples are bakelite and melamine.
(3). They are formed from bi- and tri-functional monomers.
(4). They contain covalent bonds between various linear polymer chains.
(1). They contain strong covalents bonds in their polymer chains.
(2). Examples are bakelite and melamine.
(3). They are formed from bi- and tri-functional monomers.
(4). They contain covalent bonds between various linear polymer chains.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
On which of the following properties does the coagulating power of an ion depend ?
(1). The sign of charge on the ion alone
(2). Both magnitude and sign of the charge on the ion
(3). Size of the ion alone
(4). The magnitude of the charge on the ion alone
(1). The sign of charge on the ion alone
(2). Both magnitude and sign of the charge on the ion
(3). Size of the ion alone
(4). The magnitude of the charge on the ion alone
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Iron exhibits bcc structure at room temperature. Above 900°C, it transforms to fcc structure. The ratio of density of iron at room temperature to that at 900°C (assuming molar mass and atomic radii of iron remains constant with temperature) is
(1). \(\displaystyle \frac{1}{2}\)
(2). \(\displaystyle \frac{3 \sqrt{3}}{4 \sqrt{2}}\)
(3). \(\displaystyle \frac{4 \sqrt{3}}{3 \sqrt{2}}\)
(4). \(\displaystyle \frac{\sqrt{3}}{\sqrt{2}}\)
(1). \(\displaystyle \frac{1}{2}\)
(2). \(\displaystyle \frac{3 \sqrt{3}}{4 \sqrt{2}}\)
(3). \(\displaystyle \frac{4 \sqrt{3}}{3 \sqrt{2}}\)
(4). \(\displaystyle \frac{\sqrt{3}}{\sqrt{2}}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Considering Ellingham diagram, which of the following metals can be used to reduce alumina ?
(1). Cu
(2). Mg
(3). Zn
(4). Fe
(1). Cu
(2). Mg
(3). Zn
(4). Fe
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correction factor ‘a’ to the ideal gas equation corresponds to
(1). forces of attraction between the gas molecules.
(2). electric field present between the gas molecules.
(3). volume of the gas molecules.
(4). density of the gas molecules.
(1). forces of attraction between the gas molecules.
(2). electric field present between the gas molecules.
(3). volume of the gas molecules.
(4). density of the gas molecules.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is a sink for CO?
(1). Plants
(2). Haemoglobin
(3). Micro-organisms present in the soil
(4). Oceans
(1). Plants
(2). Haemoglobin
(3). Micro-organisms present in the soil
(4). Oceans
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which is the incorrect statement ?
(1). Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal.
(2). FeO0.98 has non stoichiometric metal deficiency defect.
(3). Density decreases in case of crystals with Schottky's defect.
(4). NaCl(s) is insulator, silicon is semi-conductor, silver is conductor, quartz is piezo electric crystal.
(1). Frenkel defect is favoured in those ionic compounds in which sizes of cation and anions are almost equal.
(2). FeO0.98 has non stoichiometric metal deficiency defect.
(3). Density decreases in case of crystals with Schottky's defect.
(4). NaCl(s) is insulator, silicon is semi-conductor, silver is conductor, quartz is piezo electric crystal.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following characteristics is associated with adsorption ?
(1). ∆G, ∆H and ∆S all are negative
(2). ∆G and ∆H are negative but ∆S is positive
(3). ∆G and ∆S are negative but ∆H is positive
(4). ∆G is negative but ∆H and ∆S are positive
(1). ∆G, ∆H and ∆S all are negative
(2). ∆G and ∆H are negative but ∆S is positive
(3). ∆G and ∆S are negative but ∆H is positive
(4). ∆G is negative but ∆H and ∆S are positive
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is an analgesic ?
(1). Penicillin
(2). Streptomycin
(3). Chloromycetin
(4). Novalgin
(1). Penicillin
(2). Streptomycin
(3). Chloromycetin
(4). Novalgin
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Natural rubber has
(1). all trans - configuration
(2). alternate cis- and trans - configuration
(3). random cis- and trans - configuration
(4). all cis- configuration
(1). all trans - configuration
(2). alternate cis- and trans - configuration
(3). random cis- and trans - configuration
(4). all cis- configuration
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Equal moles of hydrogen and oxygen gases are placed in container with a pin - hole through which both can escape . What fraction of the oxygen escapes in the time required for one - half of the hydrogen to escape ?
(1). \(\displaystyle \frac{\text{1}}{\text{4}}\)
(2). \(\displaystyle \frac{\text{3}}{\text{8}}\)
(3). \(\displaystyle \frac{\text{1}}{\text{2}}\)
(4). \(\displaystyle \frac{\text{1}}{\text{8}}\)
(1). \(\displaystyle \frac{\text{1}}{\text{4}}\)
(2). \(\displaystyle \frac{\text{3}}{\text{8}}\)
(3). \(\displaystyle \frac{\text{1}}{\text{2}}\)
(4). \(\displaystyle \frac{\text{1}}{\text{8}}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Lithium has a bcc structure . Its density is 530 kg m-3 and its atomic mass is 6.94 g mol-1 . Calculate the edge length of a unit cell of lithium metal.
( NA = 6.02 × 1023 mol-1 ).
(1). 352 pm
(2). 527 pm
(3). 264 pm
(4). 154 pm
( NA = 6.02 × 1023 mol-1 ).
(1). 352 pm
(2). 527 pm
(3). 264 pm
(4). 154 pm
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The ionic radii of A + and B- ions are 0.98 × 10-10 m and 1.81 × 10-10 m . The coordination number of each ion in AB is
(1). 4
(2). 8
(3). 2
(4). 6
(1). 4
(2). 8
(3). 2
(4). 6
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
