Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 26
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
Given below are two statements:
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
(1). Statement I is true but Statement II is false.
(2). Statement I is false but Statement II is true.
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Statement I: Both \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) and \([\text{CoF}_6]^{3-}\) complexes are octahedral but differ in their magnetic behavior.
Statement II: \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is diamagnetic whereas \([\text{CoF}_6]^{3-}\) is paramagnetic .
In the light of the above statements, Choose the correct answer form the options given below
(1). Statement I is true but Statement II is false.
(2). Statement I is false but Statement II is true.
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II
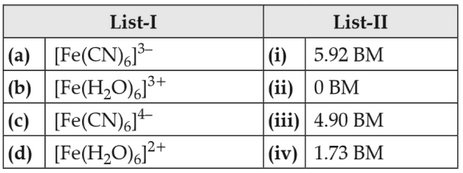
Choose the correct answer from the options given below
(1). (a)-(iv), (b)-(ii), (c)-(i), (d)-(iii)
(2). (a)-(ii), (b)-(iv), (c)-(iii), (d)-(i)
(3). (a)-(i), (b)-(iii), (c)-(iv), (d)-(ii)
(4). (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)

Choose the correct answer from the options given below
(1). (a)-(iv), (b)-(ii), (c)-(i), (d)-(iii)
(2). (a)-(ii), (b)-(iv), (c)-(iii), (d)-(i)
(3). (a)-(i), (b)-(iii), (c)-(iv), (d)-(ii)
(4). (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match the metal ions given in Column I with the spin magnetic moments of the ions given in Column II and assign the correct code

(1). (a) - (iii), (b) - (v), (c) - (i), (d) - (ii)
(2). (a) - (iv), (b) - (i), (c) - (ii), (d) - (iii)
(3). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
(4). (a) - (iv), (b) - (v), (c) - (ii), (d) - (i)

(1). (a) - (iii), (b) - (v), (c) - (i), (d) - (ii)
(2). (a) - (iv), (b) - (i), (c) - (ii), (d) - (iii)
(3). (a) - (i), (b) - (ii), (c) - (iii), (d) - (iv)
(4). (a) - (iv), (b) - (v), (c) - (ii), (d) - (i)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The geometry and magnetic behaviour of the complex [Ni(CO)4] are
(1). tetrahedral geometry and paramagnetic.
(2). square planar geometry and paramag-netic.
(3). tetrahedral geometry and diamagnetic.
(4). square planar geometry and diamagnetic.
(1). tetrahedral geometry and paramagnetic.
(2). square planar geometry and paramag-netic.
(3). tetrahedral geometry and diamagnetic.
(4). square planar geometry and diamagnetic.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Correct increasing order for the wavelengths of absorption in the visible region for the complexes of Co3+ is
(1). [Co(NH3)6 ]3+, [Co(en)3]3+, [Co(H2O)6]3+
(2). [Co(en)3)]3+, [Co(NH3)6 ]3+, [Co(H2O)6]3+
(3). [Co(H2O)6]3+, [Co(en)3)]3+, [Co(NH3)6 ]3+
(4). [Co(H2O)6]3+, [Co(NH3)6 ]3+, [Co(en)3)]3+
(1). [Co(NH3)6 ]3+, [Co(en)3]3+, [Co(H2O)6]3+
(2). [Co(en)3)]3+, [Co(NH3)6 ]3+, [Co(H2O)6]3+
(3). [Co(H2O)6]3+, [Co(en)3)]3+, [Co(NH3)6 ]3+
(4). [Co(H2O)6]3+, [Co(NH3)6 ]3+, [Co(en)3)]3+
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
HgCl2 and I 2 both when dissolved in water containing I- ions, the pair ofspecies formed is
(1). Hg2I2, I-
(2). HgI2, I3-
(3). HgI2, I-
(4). HgI42-, I3-
(1). Hg2I2, I-
(2). HgI2, I3-
(3). HgI2, I-
(4). HgI42-, I3-
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correct order of the wavelength of light absorbed by the following complexes is,
A. \([Co\left(NH_3\right)_6]^{3+}\)
B. \([Co\left(CN\right)_6]^{3-}\)
C. \([Cu\left(H_2O\right)_4]^{2+}\)
D. \([Ti \left(H_2O \right)_6 ]^{3+}\)
Choose the correct answer from the options given below:
(1). C < A < D < B
(2). B < D < A < C
(3). B < A < D < C
(4). C < D < A < B.
A. \([Co\left(NH_3\right)_6]^{3+}\)
B. \([Co\left(CN\right)_6]^{3-}\)
C. \([Cu\left(H_2O\right)_4]^{2+}\)
D. \([Ti \left(H_2O \right)_6 ]^{3+}\)
Choose the correct answer from the options given below:
(1). C < A < D < B
(2). B < D < A < C
(3). B < A < D < C
(4). C < D < A < B.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Out of the following complex compounds, which of the compound will be having the minimum conductance in solution?
(1). \([\text{Co}\left(\text{NH}_3\right)_5 CI]CI\)
(2). \([\text{Co}\left(\text{NH}_3\right)_5 CI_3]\)
(3). \([\text{Co}\left(\text{NH}_3\right)_4 CI_2]\)
(4). \([\text{Co}\left(\text{NH}_3\right)_56CI_3]\)
(1). \([\text{Co}\left(\text{NH}_3\right)_5 CI]CI\)
(2). \([\text{Co}\left(\text{NH}_3\right)_5 CI_3]\)
(3). \([\text{Co}\left(\text{NH}_3\right)_4 CI_2]\)
(4). \([\text{Co}\left(\text{NH}_3\right)_56CI_3]\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following are paramagnetic?
A. \([\text{NICI}_4]^{2-}\)
B. \(\text{Ni(CO)}_4\)
C. \([\text{NICN}_4]^{2-}\)
D. \([\text{NI(H)}_2\text{O)}_6]^{2+}\)
E. \(\text{Ni(PPh}_3\text{)}_4\)
Choose the correct answer from the options given below:
(1). A, D and E only
(2). A and C only
(3). B and E only
(4). A and D only
A. \([\text{NICI}_4]^{2-}\)
B. \(\text{Ni(CO)}_4\)
C. \([\text{NICN}_4]^{2-}\)
D. \([\text{NI(H)}_2\text{O)}_6]^{2+}\)
E. \(\text{Ni(PPh}_3\text{)}_4\)
Choose the correct answer from the options given below:
(1). A, D and E only
(2). A and C only
(3). B and E only
(4). A and D only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
During the preparation of Mohr’s salt solution (Ferrous ammonium sulphate), which of the following acid is added to prevent hydrolysis of \(Fe^{2+}\) ion
(1). Dilute nitric acid
(2). Dilute sulphuric acid
(3). Dilute hydrochloric acid
(4). Concentrated sulphuric acid
(1). Dilute nitric acid
(2). Dilute sulphuric acid
(3). Dilute hydrochloric acid
(4). Concentrated sulphuric acid
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Statement I:
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below
(1). Statement I is true but Statement II is false.
(2). Statement I is false but Statement II is true.
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
\([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) is a homoleptic complex where as \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) is a heteroleptic complex.
Statement II:
Complex \([\text{Co}\left(\text{NH}_3\right)_6]^{3+}\) has only one kind of ligands but \([\text{Co}\left(\text{NH}_3\right)_4]\text{Cl}_2]^+\) has more than one kind of ligands.
In the light of the above statements, CVhoose the correct answer from the options given below
(1). Statement I is true but Statement II is false.
(2). Statement I is false but Statement II is true.
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II
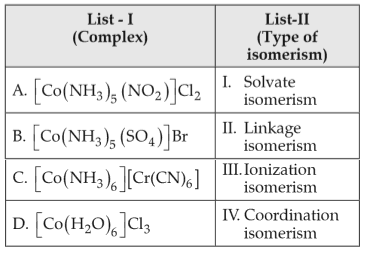
Choose the correct answer from the options given below:
(1). A - I , B - IV , C - III , D - II
(2). A - II , B - IV , C - III , D - I
(3). A - II , B - III , C - IV , D - I
(4). A - I , B - III , C - IV , D - II

Choose the correct answer from the options given below:
(1). A - I , B - IV , C - III , D - II
(2). A - II , B - IV , C - III , D - I
(3). A - II , B - III , C - IV , D - I
(4). A - I , B - III , C - IV , D - II
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Homoleptic complex from the following complex
is:
(1). Diamminechloridonitrito-N-platinum (II)
(2). Pentaamminecarbonatocobalt (III) chloride
(3). Triamminetriaquachromium (III) chloride
(4). Potassium trioxalatoaluminate (III)
(1). Diamminechloridonitrito-N-platinum (II)
(2). Pentaamminecarbonatocobalt (III) chloride
(3). Triamminetriaquachromium (III) chloride
(4). Potassium trioxalatoaluminate (III)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which complex compound is most stable?
(1).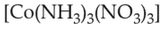
(2).
(3).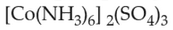
(4).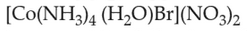
(1).
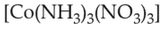
(2).

(3).
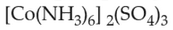
(4).
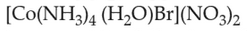
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The IUPAC name of the complex - [Ag(H2O)2] [Ag(CN)2] is :
(1). dicyanido silver(II) diaqua argentate(II)
(2). diaqua silver(II) dicyanido argentate(II)
(3). dicyanido silver(I) diaqua argentate(I)
(4). diaqua silver(I) dicyanido argentate(I)
(1). dicyanido silver(II) diaqua argentate(II)
(2). diaqua silver(II) dicyanido argentate(II)
(3). dicyanido silver(I) diaqua argentate(I)
(4). diaqua silver(I) dicyanido argentate(I)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The order of energy absorbed which is responsiblefor the color of complexes
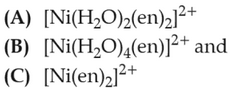
(1). [A] > [B] > [C]
(2). [C] > [B] > [A]
(3). [C] > [A]> [B]
(4). [B] > [A] > [C]
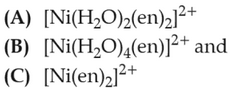
(1). [A] > [B] > [C]
(2). [C] > [B] > [A]
(3). [C] > [A]> [B]
(4). [B] > [A] > [C]
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Ethylene diaminetetraacetate (EDTA) ion is :
(1). Hexadentate ligand with four 'O and two N donor atoms
(2). Unidentate ligand
(3). Bidentate ligand with two N donor atoms
(4). Tridentate ligand with three N donor atoms
(1). Hexadentate ligand with four 'O and two N donor atoms
(2). Unidentate ligand
(3). Bidentate ligand with two N donor atoms
(4). Tridentate ligand with three N donor atoms
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Urea reacts with water to form A which will
decompose to form B. B when passed through Cu2+ (aq), deep blue colour solution C is formed. What is the formula of C from the following ?
(1). [Cu(NH3)4]2+
(2). Cu(OH)2
(3). CuCO3Cu(OH)2
(4). Cuso4
(1). [Cu(NH3)4]2+
(2). Cu(OH)2
(3). CuCO3Cu(OH)2
(4). Cuso4
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is the correct order of
increasing field strength of ligands to form
coordination compounds ?
(1). SCN- < F- < CN- < C2O42-
(2). F- < SCN- < C2O42- < CN-
(3). CN- < C2O42- < SCN- < F-
(4). SCN- < F- < C2O42- < CN-
(1). SCN- < F- < CN- < C2O42-
(2). F- < SCN- < C2O42- < CN-
(3). CN- < C2O42- < SCN- < F-
(4). SCN- < F- < C2O42- < CN-
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
What is the correct electronic configuration of the central atom in K4[Fe(CN)6] based on crystal field theory?
(1). \(\text{t}^6_{2g} \text{e}^0_g\)
(2). \(\text{e}^3 \text{t}^3_2\)
(3). \(\text{e}^4 \text{t}^2_2\)
(4). \(\text{t}^4_{2g} \text{e}^2_g\)
(1). \(\text{t}^6_{2g} \text{e}^0_g\)
(2). \(\text{e}^3 \text{t}^3_2\)
(3). \(\text{e}^4 \text{t}^2_2\)
(4). \(\text{t}^4_{2g} \text{e}^2_g\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Iron carbonyl, Fe(CO) 5 is
(1). Dinuclear
(2). Trinuclear
(3). Mononuclear
(4). Tetranuclear
(1). Dinuclear
(2). Trinuclear
(3). Mononuclear
(4). Tetranuclear
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following ions exhibits d-d transition and paramagnetism as well ?
(1). \(\text{MnO}_4^{2-}\)
(2). \(\text{MnO}_4^{-}\)
(3). \(\text{Cr}_2 \text{O}_7^{2-}\)
(4). \(\text{CrO}_4^{2-}\)
(1). \(\text{MnO}_4^{2-}\)
(2). \(\text{MnO}_4^{-}\)
(3). \(\text{Cr}_2 \text{O}_7^{2-}\)
(4). \(\text{CrO}_4^{2-}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The type of isomerism shown by the complex [CoCl2(en)2] is
(1). linkage isomerism
(2). ionization isomerism
(3). coordination isomerism
(4). geometrical isomerism
(1). linkage isomerism
(2). ionization isomerism
(3). coordination isomerism
(4). geometrical isomerism
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Pick out the correct statement with respect to [Mn(CN)6]3–
(1). It is dsp2 hybridized and square planar.
(2). It is sp3d2 hybridized and octahedral.
(3). It is sp3d2 hybridized and tetrahedral.
(4). It is d2sp3 hybridized and octahedral.
(1). It is dsp2 hybridized and square planar.
(2). It is sp3d2 hybridized and octahedral.
(3). It is sp3d2 hybridized and tetrahedral.
(4). It is d2sp3 hybridized and octahedral.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correct order of the stoichiometries of AgCl formed when AgNO3 in excess istreated with the complexes:
CoCl3.6NH3, CoCl3.5NH3, CoCl3 .4NH3 respectively is
(1). 2 AgCl, 3 AgCl, 1 AgCl
(2). 1 AgCl, 3 AgCl, 2 AgCl
(3). 3 AgCl, 1 AgCl, 2 AgCl
(4). 3 AgCl, 2 AgCl, 1 AgCl
CoCl3.6NH3, CoCl3.5NH3, CoCl3 .4NH3 respectively is
(1). 2 AgCl, 3 AgCl, 1 AgCl
(2). 1 AgCl, 3 AgCl, 2 AgCl
(3). 3 AgCl, 1 AgCl, 2 AgCl
(4). 3 AgCl, 2 AgCl, 1 AgCl
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
An example of a sigma bonded organometallic compound is
(1). cobaltocene.
(2). ruthenocene.
(3). grignard's reagent.
(4). ferrocene.
(1). cobaltocene.
(2). ruthenocene.
(3). grignard's reagent.
(4). ferrocene.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
