Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 20
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R:
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Assertion A : Metallic sodium dissolves in liquid ammonia giving a deep blue solution, which is paramagnetic.
Reason R: The deep blue solution is due to the formation of amide.
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List - I with List - Il
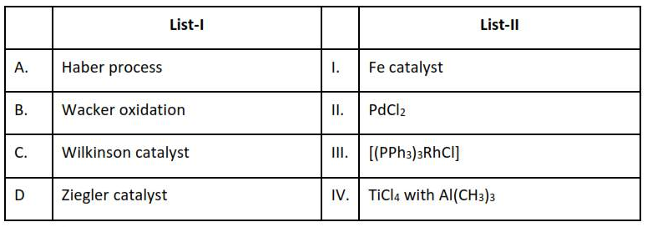
Choose the correct answer from the options given below :
(1). A-I, B-IV, C-III, D-II
(2). A-I, B-II, C-IV, D-III
(3). A-II, B-III, C-I, D-IV
(4). A-I, B-II, C-III, D-IV

Choose the correct answer from the options given below :
(1). A-I, B-IV, C-III, D-II
(2). A-I, B-II, C-IV, D-III
(3). A-II, B-III, C-I, D-IV
(4). A-I, B-II, C-III, D-IV
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements :
Statement I : Ferromagnetism is considered as an extreme form of paramagnetism.
Statement II : The number of unpaired electrons in a \(Cr^{2+}\) ion (Z=24) is the same as that of a \(Nd^{3+}\) ion (Z= 60).
In the light of the above statements, choose the correct answer from the options given below :
(1). Statement I is false but Statement II is true
(2). Both Statement I and Statement II are true
(3). Both Statement I and Statement II are false
(4). Statement I is true but Statement II is false
Statement I : Ferromagnetism is considered as an extreme form of paramagnetism.
Statement II : The number of unpaired electrons in a \(Cr^{2+}\) ion (Z=24) is the same as that of a \(Nd^{3+}\) ion (Z= 60).
In the light of the above statements, choose the correct answer from the options given below :
(1). Statement I is false but Statement II is true
(2). Both Statement I and Statement II are true
(3). Both Statement I and Statement II are false
(4). Statement I is true but Statement II is false
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are certain cations. Using inorganic qualitative analysis, arrange them in increasing group number from 0 to VI.
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
(1). E, C, D, B, A
(2). E, A, B, C, D
(3). B, A, D, C, E
(4). B, C, A, D, E
A. \(\text{Al}^{3+}\)
B. \(\text{Cu}^{2+}\)
C. \(\text{Ba}^{2+}\)
D. \(\text{Co}^{2+}\)
E. \(\text{Mg}^{2+}\)
Choose the correct answer from the options given below:
(1). E, C, D, B, A
(2). E, A, B, C, D
(3). B, A, D, C, E
(4). B, C, A, D, E
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Spin only' magnetic moment same for which of the following ions?
A. \(\text{Ti}^{3+}\)
B. \(\text{Cr}^{2+}\)
C. \(\text{Mn}^{2+}\)
D. \(\text{Fe}^{2+}\)
E. \(\text{Sc}^{3+}\)
Choose the most appropriate answer from the options given below:
(1). B and C only
(2). A and D only
(3). B and D only
(4). A and E only
A. \(\text{Ti}^{3+}\)
B. \(\text{Cr}^{2+}\)
C. \(\text{Mn}^{2+}\)
D. \(\text{Fe}^{2+}\)
E. \(\text{Sc}^{3+}\)
Choose the most appropriate answer from the options given below:
(1). B and C only
(2). A and D only
(3). B and D only
(4). A and E only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The pair of lanthanoid ions which are diamagnetic is
(1). \(\text{Gd}^{3+}\) and \(\text{Eu}^{3+}\)
(2). \(\text{Pm}^{3+}\) and \(\text{Sm}^{3+}\)
(3). \(\text{Ce}^{4+}\) and \(\text{Yb}^{2+}\)
(4). \(\text{Ce}^{3+}\) and \(\text{Eu}^{2+}\)
(1). \(\text{Gd}^{3+}\) and \(\text{Eu}^{3+}\)
(2). \(\text{Pm}^{3+}\) and \(\text{Sm}^{3+}\)
(3). \(\text{Ce}^{4+}\) and \(\text{Yb}^{2+}\)
(4). \(\text{Ce}^{3+}\) and \(\text{Eu}^{2+}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which amongst the following molecules on
polymerization produces neoprene?
(1).
(2).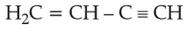
(3).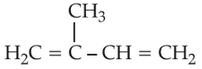
(4).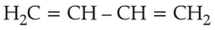
(1).

(2).
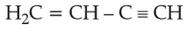
(3).
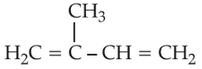
(4).
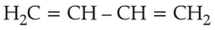
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statements are INCORRECT?
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
(1). B and D only
(2). C and D only
(3). B and C only
(4). A and E only
A. All the transition metals except scandium form MO oxides which are ionic.
B. The highest oxidation number corresponding to the group number in transition metal oxides is attained in Sc2O3 to Mn2O7.
C. Basic character increase from V2O3 to V2O4 to V2O5.
D. V2O4 dissolves in acids to give VO43- salts.
E. CrO is basic but Cr2O3 is amphoteric.
Choose the correct answer from the options given below:
(1). B and D only
(2). C and D only
(3). B and C only
(4). A and E only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Gadolinium has a low value of third ionisationenthalpy because of
(1). small size
(2). high exchange enthalpy
(3). high electronegativity
(4). high basic character
(1). small size
(2). high exchange enthalpy
(3). high electronegativity
(4). high basic character
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
In the neutral or faintly alkaline medium, KMnO4 oxidises iodide into iodate. The change in oxidation state of manganese in this reaction is from
(1). +7 to +4
(2). +6 to +4
(3). +7 to +3
(4). +6 to +5
(1). +7 to +4
(2). +6 to +4
(3). +7 to +3
(4). +6 to +5
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The RBC deficiency is deficiency disease of:
(1). Vitamin B12
(2). Vitamin B6
(3). Vitamin B1
(4). Vitamin B2
(1). Vitamin B12
(2). Vitamin B6
(3). Vitamin B1
(4). Vitamin B2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The incorrect statement among the following is :
(1). Actinoid contraction is greater for element to element than Lanthanoid contraction.
(2). Most of the trivalent Lanthanoid ions are colorless in the solid state.
(3). Lanthanoids are good conductors of heat and electricity.
(4). Actinoids are highly reactive metals, especially when finely divided.
(1). Actinoid contraction is greater for element to element than Lanthanoid contraction.
(2). Most of the trivalent Lanthanoid ions are colorless in the solid state.
(3). Lanthanoids are good conductors of heat and electricity.
(4). Actinoids are highly reactive metals, especially when finely divided.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The calculated spin only magnetic moment of Cr2+ ion is:
(1). 4.90 BM
(2). 5.92 BM
(3). 2.84 BM
(4). 3.87 BM
(1). 4.90 BM
(2). 5.92 BM
(3). 2.84 BM
(4). 3.87 BM
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
ldentify the incorrect statement.
(1). The transition metals and their compounds are known for their catalytic activity due to their ability to adopt multiple oxidation states and to form complexes.
(2). Interstitial compounds are those that are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals.
(3). The oxidation states of chromium in CrO42- and Cr2O72- are not the same.
(4). Cr2+(d4) is a stronger reducing agent than Fe2+(d6) in water.
(1). The transition metals and their compounds are known for their catalytic activity due to their ability to adopt multiple oxidation states and to form complexes.
(2). Interstitial compounds are those that are formed when small atoms like H, C or N are trapped inside the crystal lattices of metals.
(3). The oxidation states of chromium in CrO42- and Cr2O72- are not the same.
(4). Cr2+(d4) is a stronger reducing agent than Fe2+(d6) in water.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The manganate and permanganate ions are tetrahedral, due to
(1). there is no \(\pi\) -bonding.
(2). the \(\pi\) - bonding involves overlap of p-orbitals of oxygen with p-orbitals of manganese.
(3). the \(\pi\) - bonding involves overlap of d-orbitals of oxygen with d-orbitals of manganese.
(4). the \(\pi\) - bonding involves overlap of p-orbitals of oxygen with d-orbitals of manganese.
(1). there is no \(\pi\) -bonding.
(2). the \(\pi\) - bonding involves overlap of p-orbitals of oxygen with p-orbitals of manganese.
(3). the \(\pi\) - bonding involves overlap of d-orbitals of oxygen with d-orbitals of manganese.
(4). the \(\pi\) - bonding involves overlap of p-orbitals of oxygen with d-orbitals of manganese.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
It is because of inability of ns2 electrons of the valence shell to participate in bonding that
(1). Sn4+ is reducing while Pb4+ is oxidizing.
(2). Sn2+ is reducing while Pb4+ is oxidizing.
(3). Sn2+ is oxidizing while 4+is reducing.
(4). Sn2+ and Pb2+ are both oxidising and reducing.
(1). Sn4+ is reducing while Pb4+ is oxidizing.
(2). Sn2+ is reducing while Pb4+ is oxidizing.
(3). Sn2+ is oxidizing while 4+is reducing.
(4). Sn2+ and Pb2+ are both oxidising and reducing.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The reason for greater range of oxidation states in actinoids is attributed to
(1). 4f and 5d levels being close in energies.
(2). the radioactive nature of actinoids.
(3). actinoid contraction.
(4). 5f, 6d and 7s levels having comparable energies.
(1). 4f and 5d levels being close in energies.
(2). the radioactive nature of actinoids.
(3). actinoid contraction.
(4). 5f, 6d and 7s levels having comparable energies.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The element Z = 114 has been discovered recently. It will belong to which of the following family group and electronic configuration ?
(1). Nitrogen family, [Rn] 5f14 6d107s27p6
(2). Halogen family, [Rn] 5f14 6d107s27p5
(3). Carbon family, [Rn] 5f14 6d107s27p2
(4). Oxygen family, [Rn] 5f14 6d107s27p4
(1). Nitrogen family, [Rn] 5f14 6d107s27p6
(2). Halogen family, [Rn] 5f14 6d107s27p5
(3). Carbon family, [Rn] 5f14 6d107s27p2
(4). Oxygen family, [Rn] 5f14 6d107s27p4
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The electronic configurations of Eu (Atomic no. 63), Gd (Atomic no. 64) and Tb (Atomic no. 65) are
(1). [Xe] 4f6 5d1 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
(2). [Xe] 4f6 5d1 6s2, [Xe] 4f7 5d1 6s2, and [Xe] 4f8 5d1 6s2
(3). [Xe] 4f7 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
(4). [Xe] 4f7 6s2, [Xe] 4f5 6s2 and [Xe] 4f8 5d1 6s2
(1). [Xe] 4f6 5d1 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
(2). [Xe] 4f6 5d1 6s2, [Xe] 4f7 5d1 6s2, and [Xe] 4f8 5d1 6s2
(3). [Xe] 4f7 6s2, [Xe] 4f7 5d1 6s2 and [Xe] 4f9 6s2
(4). [Xe] 4f7 6s2, [Xe] 4f5 6s2 and [Xe] 4f8 5d1 6s2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following has longest C – O bond length ? ( Free C - O bond length in CO is 1.128 Å . )
(1). [ Co(CO)4]-
(2). [ Fe(CO)4]2-
(3). [ Mn(CO)6]+
(4). Ni(CO)4
(1). [ Co(CO)4]-
(2). [ Fe(CO)4]2-
(3). [ Mn(CO)6]+
(4). Ni(CO)4
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
