Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 17
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
If the molar conductivity (\(\Lambda_m\)) of a 0.050 mol L-1 solution of a monobasic weak acid is 90 S cm2 mol-1, its extent (degree) of dissociation will be
[Assume \(\Lambda_+^\circ\, =\, 349.6 \,S\, cm^2\, mol^{-1}\) and \(\Lambda_-^2\, =\, 50.4\, S\, cm^2\, mol^{-1}\)]
(1). 0.215
(2). 0.115
(3). 0.125
(4). 0.225
[Assume \(\Lambda_+^\circ\, =\, 349.6 \,S\, cm^2\, mol^{-1}\) and \(\Lambda_-^2\, =\, 50.4\, S\, cm^2\, mol^{-1}\)]
(1). 0.215
(2). 0.115
(3). 0.125
(4). 0.225
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Mass in grams of copper deposited by passing 9.6487 A current through a voltmeter containing copper sulphate solution for 100 seconds is:
(Given: Molar mass of \(\text{Cu:}\, 63\,g\,\text{mol}^{-1}\), \(1\text{F}\, =\, 96487 \text{C}\))
(1). 31.5 g
(2). 0.0315 g
(3). 3.15 g
(4). 0.315 g
(Given: Molar mass of \(\text{Cu:}\, 63\,g\,\text{mol}^{-1}\), \(1\text{F}\, =\, 96487 \text{C}\))
(1). 31.5 g
(2). 0.0315 g
(3). 3.15 g
(4). 0.315 g
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II:
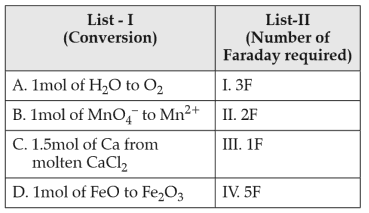
Choose the correct answer from the options given below:
(1). A-II, B-III, C-I, D-IV
(2). A-III, B-IV, C-II, D-I
(3). A-II, B-IV, C-I, D-III
(4). A-III, B-IV, C-I, D-II

Choose the correct answer from the options given below:
(1). A-II, B-III, C-I, D-IV
(2). A-III, B-IV, C-II, D-I
(3). A-II, B-IV, C-I, D-III
(4). A-III, B-IV, C-I, D-II
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The conductivity of centimolar solution of KCl at
\(25^\circ C\) is 0.0210 ohm-1 and cm-1 and the resistance
of the cell containing the solution at \(25^\circ C\) is 60 ohm. The value of cell constant is-
(1). 3.28 cm-1
(2). 1.26 cm-1
(3). 3.34 cm-1
(4). 1.35 cm-1
(1). 3.28 cm-1
(2). 1.26 cm-1
(3). 3.34 cm-1
(4). 1.35 cm-1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are half cell reactions:
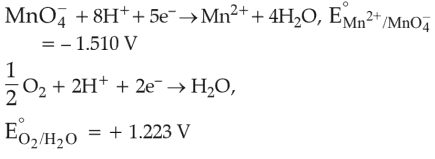
Will the permanganate ion, MnO4- liberate O2 from water in the presence of an acid?
(1). \(\text{Yes, because}\, E^\circ _{cell}\,=\,+0.287\, \text{V}\)
(2). \(\text{No, because}\, E^\circ _{cell}\,=\,-0.287\, \text{V}\)
(3). \(\text{Yes, because} \,E^\circ _{cell}\,=\,+2.733\, \text{V}\)
(4). \(\text{Np, because}\, E^\circ _{cell}\,=\,-2.733\, \text{V}\)
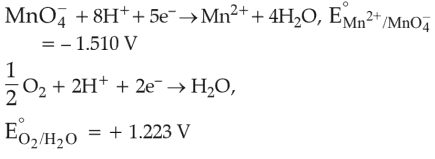
Will the permanganate ion, MnO4- liberate O2 from water in the presence of an acid?
(1). \(\text{Yes, because}\, E^\circ _{cell}\,=\,+0.287\, \text{V}\)
(2). \(\text{No, because}\, E^\circ _{cell}\,=\,-0.287\, \text{V}\)
(3). \(\text{Yes, because} \,E^\circ _{cell}\,=\,+2.733\, \text{V}\)
(4). \(\text{Np, because}\, E^\circ _{cell}\,=\,-2.733\, \text{V}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
At 298 K, the standard electrode potentials of
\(\displaystyle \frac{Cu^{2+}}{Cu}\), \(\displaystyle \frac{Zn^{2+}}{Zn}\), \(\displaystyle \frac{Fe^{2+}}{Fe}\), and \(\displaystyle \frac{Ag^{+}}{Ag}\) are \(0.34\, \text{V}\), \(-0.76\, \text{V}\), \(-0.44\, \text{ V}\) and \(0.80\, \text{V}\), respectively.
On the basis of standard electrode potential, predict which of the following reaction cannot occur?
(1).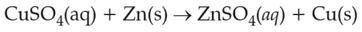
(2).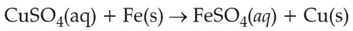
(3).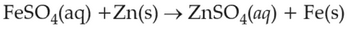
(4).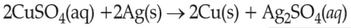
\(\displaystyle \frac{Cu^{2+}}{Cu}\), \(\displaystyle \frac{Zn^{2+}}{Zn}\), \(\displaystyle \frac{Fe^{2+}}{Fe}\), and \(\displaystyle \frac{Ag^{+}}{Ag}\) are \(0.34\, \text{V}\), \(-0.76\, \text{V}\), \(-0.44\, \text{ V}\) and \(0.80\, \text{V}\), respectively.
On the basis of standard electrode potential, predict which of the following reaction cannot occur?
(1).
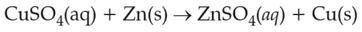
(2).
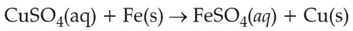
(3).
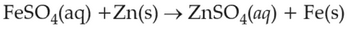
(4).
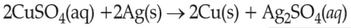
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Find the emf of the cell in which the followingreaction takes place at 298 K

(1). 1.0385 V
(2). 1.385 V
(3). 0.9615 V
(4). 1.05 V

(1). 1.0385 V
(2). 1.385 V
(3). 0.9615 V
(4). 1.05 V
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The molar conductance of NaCl, HCI and
CH3COONa at infinite dilution are 126.45, 426.16 and 91.0 S cm2 mol-1 respectively. The molar conductance of CH3COOH at infinite dilution is.
Choose the right option for your answer.
(1). 201.28 S cm2 mol-1
(2). 390.71 S cm2 mol-1
(3). 698.28 S cm2 mol-1
(4). 540.48 S cm2 mol-1
Choose the right option for your answer.
(1). 201.28 S cm2 mol-1
(2). 390.71 S cm2 mol-1
(3). 698.28 S cm2 mol-1
(4). 540.48 S cm2 mol-1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol-1, What is the dissociation constant of acetic acid ? Choose the correct option.
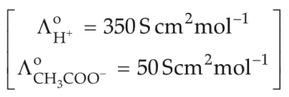
(1). 1.75 x 10-4 mol L-1
(2). 2.50 x 10-4 mol L-1
(3). 1.75 x 10-5 mol L-1
(4). 2.50 x 10-5 mol L-1
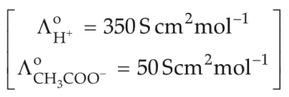
(1). 1.75 x 10-4 mol L-1
(2). 2.50 x 10-4 mol L-1
(3). 1.75 x 10-5 mol L-1
(4). 2.50 x 10-5 mol L-1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
On electrolysis of dilute suiphuric acid using Platinum
(Pt) electrode, the product obtained at anode will be:
(1). Oxygen gas
(2). H2S gas
(3). SO2 gas
(4). Hydrogen gas
(1). Oxygen gas
(2). H2S gas
(3). SO2 gas
(4). Hydrogen gas
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The number of Faradays(F) required to produce
20 g of calcium from molten CaCl2 (Atomic mass of Ca = 40 g mol-1) is:
(1). 2
(2). 3
(3). 4
(4). 1
(1). 2
(2). 3
(3). 4
(4). 1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
For a cell involving one electron \(\text{E}^\circ _\text{cell}\) = 0.59 V at 298 K, the equilibrium constant for the cell reaction is
[Given that \(\displaystyle \frac{2.303 RT}{F}\) = 0.059 V at T = 298 K]
(1). 1.0 x 105
(2). 1.0 x 1010
(3). 1.0 x 1030
(4). 1.0 x 102
[Given that \(\displaystyle \frac{2.303 RT}{F}\) = 0.059 V at T = 298 K]
(1). 1.0 x 105
(2). 1.0 x 1010
(3). 1.0 x 1030
(4). 1.0 x 102
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
For the cell reaction
2Fe3+(aq) + 2I–(aq) \(\rightarrow\) 2Fe2+(aq) + I2 (aq)
\(\text{E} ^\oplus_\text{cell}\) = 0.24 V at 298 K. The standard Gibbs energy cell (\(\Delta_r \text{G}^\ominus\)) of the cell reaction is
[Given that Faraday constant F = 96500 C mol–1]
(1). – 23.16 kJ mol–1
(2). 46.32 kJ mol–1
(3). 23.16 kJ mol–1
(4). – 46.32 kJ mol–1
2Fe3+(aq) + 2I–(aq) \(\rightarrow\) 2Fe2+(aq) + I2 (aq)
\(\text{E} ^\oplus_\text{cell}\) = 0.24 V at 298 K. The standard Gibbs energy cell (\(\Delta_r \text{G}^\ominus\)) of the cell reaction is
[Given that Faraday constant F = 96500 C mol–1]
(1). – 23.16 kJ mol–1
(2). 46.32 kJ mol–1
(3). 23.16 kJ mol–1
(4). – 46.32 kJ mol–1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Extraction of gold and silver involves leaching with CN- ion. Silver is later recovered by
(1). Displacement with Zn
(2). Liquation
(3). Distillation
(4). Zone refining
(1). Displacement with Zn
(2). Liquation
(3). Distillation
(4). Zone refining
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Ionic mobility of which of the following alkali metal ions is lowest when aqueous solution of their salts are put under an electric field ?
(1). Li
(2). Na
(3). K
(4). Rb
(1). Li
(2). Na
(3). K
(4). Rb
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
In the electrochemical cell
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)
(1). E2 = 0 \(\neq\) E1
(2). E1 = E2
(3). E1 < E2
(4). E1 > E2
Zn|ZnSO4 (0.01M)| |CuSO4(1.0 M)|Cu, the emf of this Daniel cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the following, which one is relationship between E1 and E2
(Given, \(\frac{\text{RT}}{\text{F}}\) = 0.059)
(1). E2 = 0 \(\neq\) E1
(2). E1 = E2
(3). E1 < E2
(4). E1 > E2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The pressure ofH2 required to make the potential of H<2/sub> - electrode zero in pure water at 298 K is
(1). 10-12 atm
(2). 10-10 atm
(3). 10-4 atm
(4). 10-14 atm
(1). 10-12 atm
(2). 10-10 atm
(3). 10-4 atm
(4). 10-14 atm
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
