Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 19
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
Given below are two statements: one is labelled
as Assertion A and the other is labelled as
Reason R :
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Assertion A: Helium is used to dilute oxygen in diving apparatus.
Reason R: Helium has high solubility in O2
In the light of the above statements, choose the correct answer from the options given below:
(1). Both A and R are true but R is NOT the correct explanation of A.
(2). A is true but R is false.
(3). A is false but R is true.
(4). Both A and R are true and R is the correct explanation of A.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following aqueous solution will exhibit highest boiling point?
(1). \(\text{0.015M}\,C_6H_12O_6\)
(2). \(\text{0.01M Urea}\)
(3). \(\text{0.01M}\, KNO_3\)
(4). \(\text{0.01M}\, Na_2SO_4\)
(1). \(\text{0.015M}\,C_6H_12O_6\)
(2). \(\text{0.01M Urea}\)
(3). \(\text{0.01M}\, KNO_3\)
(4). \(\text{0.01M}\, Na_2SO_4\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List - I with List - II
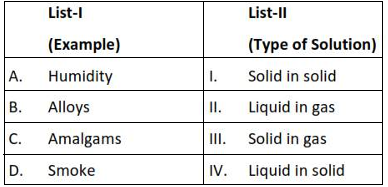
Choose the correct answer from the options given below :
(1). A-III, B-II, C-I, D-IV
(2). A-II, B-IV, C-I, D-III
(3). A-II, B-I, C-IV, D-III
(4). A-III, B-I, C-IV, D-II

Choose the correct answer from the options given below :
(1). A-III, B-II, C-I, D-IV
(2). A-II, B-IV, C-I, D-III
(3). A-II, B-I, C-IV, D-III
(4). A-III, B-I, C-IV, D-II
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
5 moles of liquid X and 10 moles of liquid Y make a solution having a vapour pressure of 70 torr. The vapour pressures of pure X and Y are 63 torr and 78 torr respectively, Which of the following is true regarding the described solution?
(1). The solution has volume greater than the sum of individual volumes.
(2). The solution shows positive deviation.
(3). The solution shows negative deviation.
(4). The solution is ideal.
(1). The solution has volume greater than the sum of individual volumes.
(2). The solution shows positive deviation.
(3). The solution shows negative deviation.
(4). The solution is ideal.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The plot of osmotic pressure (\(\pi\)) vs concentration (\(\text{mol L}^{-1}\)) for a solution gives a straight line with slope \(25.73\,\text{L bar mol}^{-1}\). The temperature at which the osmotic pressure measurement is done is
(Use \(\text{R}\,=\, 0.083\,\text{L bar mol}^{-1}\text{K}^{-1}\))
(1). \(25.73^\circ\,\text{C}\)
(2). \(12.05^\circ\,\text{C}\)
(3). \(37^\circ\,\text{C}\)
(4). \(310^\circ\,\text{C}\)
(Use \(\text{R}\,=\, 0.083\,\text{L bar mol}^{-1}\text{K}^{-1}\))
(1). \(25.73^\circ\,\text{C}\)
(2). \(12.05^\circ\,\text{C}\)
(3). \(37^\circ\,\text{C}\)
(4). \(310^\circ\,\text{C}\)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The Henry's law constant \(\left(\text{K}_\text{H}\right)\) values of three gases (A, B, C) in water are \(145\), \(2 \times 10^{-5}\) and \(35\) kbar, respectively. The solubility of these gases in water follow the order:
(1). A > C > B
(2). A > B > C
(3). B > A > C
(4). B > C > A
(1). A > C > B
(2). A > B > C
(3). B > A > C
(4). B > C > A
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The following solutions were prepared by dissolving 10 g of glucose (C6H12O6) in 250 ml of water (P1), 10 gof urea (CH4N2O) in 250 ml of water (P2) and 10 g of sucrose (C12H22O11) in 250 ml of water (P3). The right option for the decreasing order of osmotic pressure of these solutions is :
(1). P2 > P1 > P3
(2). P1 > P2 > P3
(3). P2 > P3 > P1
(4). P3 > P1 > P2
(1). P2 > P1 > P3
(2). P1 > P2 > P3
(3). P2 > P3 > P1
(4). P3 > P1 > P2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correct option for the value of vapour pressure of a solution at 45°C with benzene to octane in molar ratio 3: 2 is :
[At 45°C vapour pressure of benzene is 280 mm Hg and that of octane is 420 mm Hg, Assume Ideal gas]
(1). 160 mm of Hg
(2). 168 mm of Hg
(3). 336 mm of Hg
(4). 350 mm of Hg
[At 45°C vapour pressure of benzene is 280 mm Hg and that of octane is 420 mm Hg, Assume Ideal gas]
(1). 160 mm of Hg
(2). 168 mm of Hg
(3). 336 mm of Hg
(4). 350 mm of Hg
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The mixture which shows positive deviation from
Raoult's law is:
(1). Benzene + Toluene
(2). Acetone + Chloroform
(3). Chloroethane + Bromoethane
(4). Ethanol+Acetone
(1). Benzene + Toluene
(2). Acetone + Chloroform
(3). Chloroethane + Bromoethane
(4). Ethanol+Acetone
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Find out the solubility of Ni(OH)2 in 0.1 M NaOH. Given that the ionic product of Ni(OH)2 is 2 x 10-15
(1). 2 x 10-8 M
(2). 1 x 10-13 M
(3). 1 x 108 M
(4). 2 x 10-13 M
(1). 2 x 10-8 M
(2). 1 x 10-13 M
(3). 1 x 108 M
(4). 2 x 10-13 M
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The freezing point depression constant (Kf) of benzene is 5.12 K kg mol-1. The freezing point depression for the solution of molality 0.078 m containing a non-electrolyte solute in benzene is
(rounded off up to two decimal places):
(1). 0.80 K
(2). 0.40 K
(3). 0.60 K
(4). 0.20 K
(rounded off up to two decimal places):
(1). 0.80 K
(2). 0.40 K
(3). 0.60 K
(4). 0.20 K
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
HCl was passed through a solution of CaCI2, MgCi2 and NaCI. Which of the following compound(s) crystallise(s)?
(1). Only NaCI
(2). Only MgCl2
(3). NaCI, MgCl2 and CaCl2
(4). Both MgCl2 and CaCl2
(1). Only NaCI
(2). Only MgCl2
(3). NaCI, MgCl2 and CaCl2
(4). Both MgCl2 and CaCl2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The mixture that forms maximum boiling azeotrope is
(1). Ethanol + Water
(2). Acetone + Carbon disulphide
(3). Heptane + Octane
(4). Water + Nitric acid
(1). Ethanol + Water
(2). Acetone + Carbon disulphide
(3). Heptane + Octane
(4). Water + Nitric acid
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
For an ideal solution, the correct option is
(1). \(\Delta_{mix}\) V \(\neq\) 0 at constant T and P
(2). \(\Delta_{mix}\) H = 0 at constant T and P
(3). \(\Delta_{mix}\) G = 0 at constant T and P
(4). \(\Delta_{mix}\) S = 0 at constant T and P
(1). \(\Delta_{mix}\) V \(\neq\) 0 at constant T and P
(2). \(\Delta_{mix}\) H = 0 at constant T and P
(3). \(\Delta_{mix}\) G = 0 at constant T and P
(4). \(\Delta_{mix}\) S = 0 at constant T and P
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
For a given reaction, \(\Delta \text{H}\) = 35.5 kJ mol–1 and \(\Delta \text{S}\) = 83.6 JK–1 mol–1. The reaction is spontaneous at :
[Assume that \(\Delta \text{H}\) and \(\Delta \text{S}\) do not vary with temperature]
(1). T > 298 K
(2). T < 425 K
(3). T > 425 K
(4). All temperatures
[Assume that \(\Delta \text{H}\) and \(\Delta \text{S}\) do not vary with temperature]
(1). T > 298 K
(2). T < 425 K
(3). T > 425 K
(4). All temperatures
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
If molality of the dilute solution is doubled, the value of molal depression constant (Kf ) will be
(1). unchanged.
(2). doubled.
(3). halved.
(4). tripled.
(1). unchanged.
(2). doubled.
(3). halved.
(4). tripled.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following is dependent on temperature ?
(1). Weight percentage
(2). Molality
(3). Molarity
(4). Mole fraction
(1). Weight percentage
(2). Molality
(3). Molarity
(4). Mole fraction
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
At 100°C the vapour pressure of a solution of 6.5 g of a solute in 100 g water is 732 mm. If Kb = 0.52, the boiling point of this solution will be
(1). 100°C
(2). 102°C
(3). 103°C
(4). 101°C
(1). 100°C
(2). 102°C
(3). 103°C
(4). 101°C
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statements about the composition of the vapour over an ideal 1 : 1 molar mixture of benzene and toluene is correct ? Assume that the temperature is constant at 25 ° C.
( Given , vapour pressure data at 25 ° C , benzene = 12.8 kPa , toluene = 3.85 kPa )
(1). The vapour will contain a higher percentage of toluene
(2). The vapour will contain equal amounts of benzene and toluene
(3). Not enough information is given to make a prediction
(4). The vapour will contain a higher percentage of benzene
( Given , vapour pressure data at 25 ° C , benzene = 12.8 kPa , toluene = 3.85 kPa )
(1). The vapour will contain a higher percentage of toluene
(2). The vapour will contain equal amounts of benzene and toluene
(3). Not enough information is given to make a prediction
(4). The vapour will contain a higher percentage of benzene
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
