Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 34
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
The \(\text{E}^\circ\) value for the \(\text{Mn}^{3+}\,/\,\text{Mn}^{2+}\) couple is more positive than that of \(\text{Cr}^{3+}\,/\,\text{Cr}^{2+}\) or \(\text{Fe}^{3+}\,/\,\text{Fe}^{2+}\) due to change of:
(1). \(d^4\) to \(d^5\) configuration
(2). \(d^3\) to \(d^5\) configuration
(3). \(d^5\) to \(d^4\) configuration
(4). \(d^5\) to \(d^2\) configuration
(1). \(d^4\) to \(d^5\) configuration
(2). \(d^3\) to \(d^5\) configuration
(3). \(d^5\) to \(d^4\) configuration
(4). \(d^5\) to \(d^2\) configuration
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List -I with List - II.
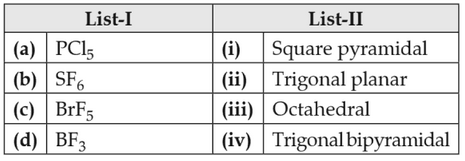
Choose the correct answer from the options given below.
(1). (a)-(iv), (b)-(iii), (c)-(i), (d)-(ii)
(2). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(3). (a)-(iii), (b)-(i), (c)-(iv), (d)-(ii)
(4). (a)-(iv), (b)-(iii), (c)-(i), (d)-(i)

Choose the correct answer from the options given below.
(1). (a)-(iv), (b)-(iii), (c)-(i), (d)-(ii)
(2). (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(3). (a)-(iii), (b)-(i), (c)-(iv), (d)-(ii)
(4). (a)-(iv), (b)-(iii), (c)-(i), (d)-(i)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following molecules is non-polar in nature?
(1). POCl3
(2). CH2O
(3). SbCl5
(4). NO2
(1). POCl3
(2). CH2O
(3). SbCl5
(4). NO2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The number of sigma (\(\sigma\)) and pi (\(\pi\)) bonds in pent-2 en-4-yne is
(1). 8 \(\sigma\) bonds and 5 \(\pi\) bonds
(2). 11 \(\sigma\) bonds and 2 \(\pi\) bonds
(3). 13 \(\sigma\) bonds and no \(\pi\) bonds
(4). 10 \(\sigma\) bonds and 3 \(\pi\) bonds
(1). 8 \(\sigma\) bonds and 5 \(\pi\) bonds
(2). 11 \(\sigma\) bonds and 2 \(\pi\) bonds
(3). 13 \(\sigma\) bonds and no \(\pi\) bonds
(4). 10 \(\sigma\) bonds and 3 \(\pi\) bonds
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
In the structure of ClF3, the number of lone pair of electrons on central atom ‘Cl’ is
(1). Three
(2). Four
(3). Two
(4). One
(1). Three
(2). Four
(3). Two
(4). One
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following molecules represents the order of hybridization sp2, sp2, sp, sp from left to right atoms ?
(1). CH3 – CH = CH – CH3
(2). CH2 = CH – CH = CH2
(3). CH2 = CH – C ≡ CH
(4). HC ≡ C – C ≡ CH
(1). CH3 – CH = CH – CH3
(2). CH2 = CH – CH = CH2
(3). CH2 = CH – C ≡ CH
(4). HC ≡ C – C ≡ CH
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match the interhalogen compounds of column I with the geometry in column II and assign the correct code.
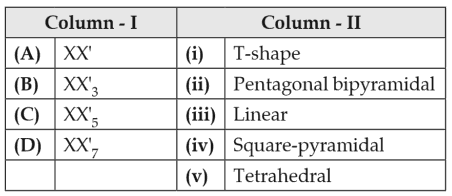
(1). (a) - (iv), (b) - (iii), (c) - (ii), (d) - (i)
(2). (a) - (iii), (b) - (iv), (c) - (i), (d) - (ii)
(3). (a) - (iii), (b) - (i), (c) - (iv), (d) - (ii)
(4). (a) - (v), (b) - (iv), (c) - (iii), (d) - (ii)

(1). (a) - (iv), (b) - (iii), (c) - (ii), (d) - (i)
(2). (a) - (iii), (b) - (iv), (c) - (i), (d) - (ii)
(3). (a) - (iii), (b) - (i), (c) - (iv), (d) - (ii)
(4). (a) - (v), (b) - (iv), (c) - (iii), (d) - (ii)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which one of the following pairs of species have the same bond order ?
(1). N2, O2–
(2). CO, NO
(3). O2, NO+
(4). CN-, CO
(1). N2, O2–
(2). CO, NO
(3). O2, NO+
(4). CN-, CO
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements :
Statement I : A hypothetical diatomic molecule with bond order zero is quite stable.
Statement II : As bond order increases, the bond length increases.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1). Statement I is false but Statement II is true
(2). Both Statement I and Statement II are true
(3). Both Statement I and Statement II are false
(4). Statement I is true but Statement II is false
Statement I : A hypothetical diatomic molecule with bond order zero is quite stable.
Statement II : As bond order increases, the bond length increases.
In the light of the above statements, choose the most appropriate answer from the options given below :
(1). Statement I is false but Statement II is true
(2). Both Statement I and Statement II are true
(3). Both Statement I and Statement II are false
(4). Statement I is true but Statement II is false
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Among the given compounds I - III, the correct order of bond dissociation energy of C-H bond marked with * is :
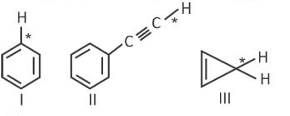
(1). II > III > I
(2). II > I > III
(3). I > II > III
(4). III > II > I

(1). II > III > I
(2). II > I > III
(3). I > II > III
(4). III > II > I
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Identify the correct orders against the property mentioned
A.\(H_2O\, >\, NH_3 \,> \,CHCI_3\) - dipole moment
B. \(XeF_4\, >\, XeO_3\, >\, XeF_2\) - number of lone pairs on central atom
C. \(O-H \,>\, C-H \,>\, N-O \) - bond length
D. \(N_2 \,>\, O_2 \,>\, H_2\) - bond enthalpy
Choose the correct answer fromthe options given below:
(1). B, C only
(2). A, D only
(3). B, D only
(4). A, C only
A.\(H_2O\, >\, NH_3 \,> \,CHCI_3\) - dipole moment
B. \(XeF_4\, >\, XeO_3\, >\, XeF_2\) - number of lone pairs on central atom
C. \(O-H \,>\, C-H \,>\, N-O \) - bond length
D. \(N_2 \,>\, O_2 \,>\, H_2\) - bond enthalpy
Choose the correct answer fromthe options given below:
(1). B, C only
(2). A, D only
(3). B, D only
(4). A, C only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Identify the correct answer
(1). Dipole moment of \(\text{NF}_3\) is greater than that of \(\text{NH}_3\)
(2). Three canonical forms can be drawn from \(\text{CO}^{2-}_3\) ion
(3). Three resonace structures can be drawn for ozone
(4). \(\text{BF}_3\) has non-zero dipole moment
(1). Dipole moment of \(\text{NF}_3\) is greater than that of \(\text{NH}_3\)
(2). Three canonical forms can be drawn from \(\text{CO}^{2-}_3\) ion
(3). Three resonace structures can be drawn for ozone
(4). \(\text{BF}_3\) has non-zero dipole moment
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Arrange the following elements in increasing order of first ionization enthalpy:
Li, Be, B, N
Choose the correct answer from the options given below:
(1). Li < Be < C < B < N
(2). Li < Be < N < B < C
(3). Li < Be < B < C < N
(4). Li < B < Be < C < N
Li, Be, B, N
Choose the correct answer from the options given below:
(1). Li < Be < C < B < N
(2). Li < Be < N < B < C
(3). Li < Be < B < C < N
(4). Li < B < Be < C < N
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II:
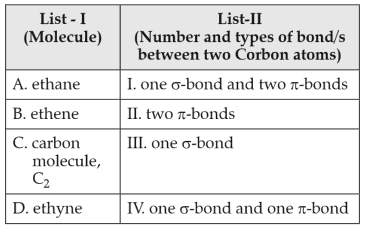
Choose the correct answer from the options given below:
(1). A-III, B-IV, C-II, D-I
(2). A-III, B-IV, C-I, D-II
(3). A-I, B-IV, C-II, D-III
(4). A-IV, B-II, C-II, D-I

Choose the correct answer from the options given below:
(1). A-III, B-IV, C-II, D-I
(2). A-III, B-IV, C-I, D-II
(3). A-I, B-IV, C-II, D-III
(4). A-IV, B-II, C-II, D-I
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Amongst the following, the total number of
species NƠT having eight electrons around central
atom in its outer most shell, is
NH3, AICI3, BeCl2, CCl4, PCI 5 :
(1). 2
(2). 4
(3). 1
(4). 3
(1). 2
(2). 4
(3). 1
(4). 3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The correct order of energies of molecular orbitals
of N2 molecule, is
(1).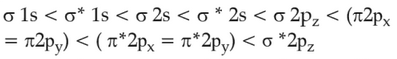
(2).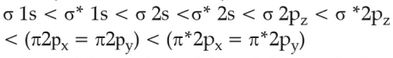
(3).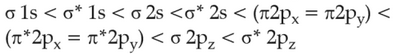
(4).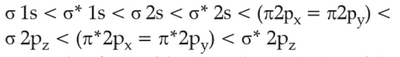
(1).
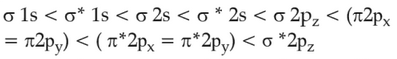
(2).
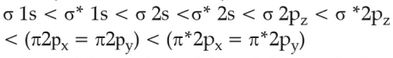
(3).
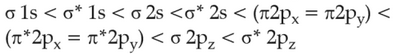
(4).
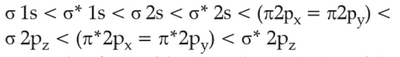
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The number of \(\sigma\) bonds, \(\pi\) bonds and lone pair of electrons in pyridine, respectively are:
(1). 12, 3,0
(2). 11, 3, 1
(3). 12, 2, 1
(4). 11, 2, 0
(1). 12, 3,0
(2). 11, 3, 1
(3). 12, 2, 1
(4). 11, 2, 0
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The stability of Cu is more than Cu+ salts in aqueous solution due to
(1). enthalpy of atomization.
(2). hydration energy.
(3). second ionisation enthalpy.
(4). first ionisation enthalpy.
(1). enthalpy of atomization.
(2). hydration energy.
(3). second ionisation enthalpy.
(4). first ionisation enthalpy.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which amongst the following is incorrect statement?
(1). The bond order of \(O_{2}^{+}\), \(O_2\), \(O_{2}^{-}\), and \(O_{2}^{2-}\) are2.5, 2, 1.5 and 1, respectively
(2). \(C_2\) molecule has four electrons in its two degenerate molecular orbitals
(3). \(H_{2}^{+}\) ion has one electron
(4). \(O_{2}^{+}\) ion is diamagnetic dry
(1). The bond order of \(O_{2}^{+}\), \(O_2\), \(O_{2}^{-}\), and \(O_{2}^{2-}\) are2.5, 2, 1.5 and 1, respectively
(2). \(C_2\) molecule has four electrons in its two degenerate molecular orbitals
(3). \(H_{2}^{+}\) ion has one electron
(4). \(O_{2}^{+}\) ion is diamagnetic dry
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Amongst the following which one will havemaximum 'lone pair - lone pair' electron repulsions?
(1). CIF3
(2). IF5
(3). SF4
(4). XeF2
(1). CIF3
(2). IF5
(3). SF4
(4). XeF2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
BF3 is planar and electron deficient compound. Hybridization and number of electrons around the central atom, respectively are:
(1). sp3 and 4
(2). sp3 and 6
(3). sp2 and 6
(4). sp2 and 8
(1). sp3 and 4
(2). sp3 and 6
(3). sp2 and 6
(4). sp2 and 8
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The structures of beryllium chloride in solid state and vapour phase, are:
(1). Chain and dimer, respectively
(2). Linear in both
(3). Dimer and Linear, respectively
(4). Chain in both
(1). Chain and dimer, respectively
(2). Linear in both
(3). Dimer and Linear, respectively
(4). Chain in both
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
From the following pairs of ions which one is not an iso-electronic pair?
(1). O2-,F-
(2). Na+,Mg2+
(3). Mn2+,Fe3+
(4). Fe2+,Mn2+
(1). O2-,F-
(2). Na+,Mg2+
(3). Mn2+,Fe3+
(4). Fe2+,Mn2+
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following set of molecules will have
zero dipole moment?
(1). Boron trifluoride, hydrogen fluoride, carbon dioxide, 1,3-dichlorobenzene
(2). Nitrogen trifluoride, beryllium difluoride, water, 1,3-dichlorobenzene
(3). Boron trifluoride, beryllium difluoride, carbon dioxide, 1,4-dichlorobenzene bn
(4). Ammonia, berylium difluoride, water, 1,4- dichlorobenzene
(1). Boron trifluoride, hydrogen fluoride, carbon dioxide, 1,3-dichlorobenzene
(2). Nitrogen trifluoride, beryllium difluoride, water, 1,3-dichlorobenzene
(3). Boron trifluoride, beryllium difluoride, carbon dioxide, 1,4-dichlorobenzene bn
(4). Ammonia, berylium difluoride, water, 1,4- dichlorobenzene
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Identify a molecule which does not exist.
(1). Li2
(2). C2
(3). O2
(4). He2
(1). Li2
(2). C2
(3). O2
(4). He2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Identify the incorrect statement related to PCl5 from the following
(1). Two axial P—Cl bonds make an angle of 180° with each other.
(2). Axial P—Cl bonds are longer than equatorial P—Cl bonds.
(3). PCl5 molecule is non-reactive.
(4). Three equatorial P—Cl bonds make an angle of 120° with each other.
(1). Two axial P—Cl bonds make an angle of 180° with each other.
(2). Axial P—Cl bonds are longer than equatorial P—Cl bonds.
(3). PCl5 molecule is non-reactive.
(4). Three equatorial P—Cl bonds make an angle of 120° with each other.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Consider the following species:
CN+, CN–, NO and CN
Which one of these will have the highest bond order ?
(1). CN
(2). CN+
(3). CN–
(4). NO
CN+, CN–, NO and CN
Which one of these will have the highest bond order ?
(1). CN
(2). CN+
(3). CN–
(4). NO
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The bond disssociation energies of X2, Y2 and XY are in the ratio 1 : 0.5 : 1. \(\Delta\)H for the formation of XY is -200 KJ mol-. The bond dissociation energy of X2 will be
(1). 400 kJ mol–1
(2). 800 kJ mol–1
(3). 100 kJ mol–1
(4). 200 kJ mol–1
(1). 400 kJ mol–1
(2). 800 kJ mol–1
(3). 100 kJ mol–1
(4). 200 kJ mol–1
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following pair of compounds is isoelectronic and isostructural ?
(1). IF3 , XeF2
(2). BeCl2, XeF2
(3). Tel2, XeF2
(4). IBr2- , XeF2
(1). IF3 , XeF2
(2). BeCl2, XeF2
(3). Tel2, XeF2
(4). IBr2- , XeF2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
With respect to the conformers of ethane, which of the following statements is true ?
(1). Both bond angles and bond length remains same.
(2). Bond angle remains same but bond length changes.
(3). Bond angle changes but bond length remains same.
(4). Both bond angle and bond length change.
(1). Both bond angles and bond length remains same.
(2). Bond angle remains same but bond length changes.
(3). Bond angle changes but bond length remains same.
(4). Both bond angle and bond length change.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The species, having bond angles of 120° is
(1). BCl3
(2). PH3
(3). ClF3
(4). NCl3
(1). BCl3
(2). PH3
(3). ClF3
(4). NCl3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The pair of electron in the given carbanion, CH3C≡C–, is present in which orbitals ?
(1). sp3
(2). sp2
(3). sp
(4). 2p
(1). sp3
(2). sp2
(3). sp
(4). 2p
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Consider the molecules CH4, NH3 and H2O. Which of the given statements is false ?
(1). The H–O–H bond angle in H2O is larger than the H–C–H bond angle in CH4
(2). the The H–O–H bond angle in H2O is smaller than H–N–H bond angle in NH3
(3). The H–C–H bond angle in CH4 is larger than the H–N–H bond angle in NH3
(4). The H–C–H bond angle in CH4, the H-N-H bond angle in NH3 and the H-O-H bond angle in H2O are all greater than 90°
(1). The H–O–H bond angle in H2O is larger than the H–C–H bond angle in CH4
(2). the The H–O–H bond angle in H2O is smaller than H–N–H bond angle in NH3
(3). The H–C–H bond angle in CH4 is larger than the H–N–H bond angle in NH3
(4). The H–C–H bond angle in CH4, the H-N-H bond angle in NH3 and the H-O-H bond angle in H2O are all greater than 90°
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Predict the correct order among the following .
(1). lone pair - lone pair > bond pair - bond pair > lone pair - bond pair
(2). bond pair - bond pair > lone pair - bond pair > lone pair - lone pair
(3). lone pair - bond pair > bond pair - bond pair > lone pair - lone pair
(4). lone pair - lone pair > lone pair - bond pair > bond pair - bond pair
(1). lone pair - lone pair > bond pair - bond pair > lone pair - bond pair
(2). bond pair - bond pair > lone pair - bond pair > lone pair - lone pair
(3). lone pair - bond pair > bond pair - bond pair > lone pair - lone pair
(4). lone pair - lone pair > lone pair - bond pair > bond pair - bond pair
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
