Home >> My Performance >> My Topic Performance >> My Question Performance
My Question Performance Summary in Topic Wise Tests !
Questions Available: 24
Questions Attempted: 15
Number of Attempts: 25
Correct Attempts: 12
Total Time Spent: 00:45
Avg Time Per Question: 00:02
My Question Performance Details in Topic Wise Tests
Match List I with List Il
Choose the correct answer from the options given below :
(1). A-III, B-II, C-I, D-IV
(2). A-III, B-IV, C-II, D-I
(3). A-III, B-IV, C-I, D-II
(4). A-III, B-II, C-IV, D-I

Choose the correct answer from the options given below :
(1). A-III, B-II, C-I, D-IV
(2). A-III, B-IV, C-II, D-I
(3). A-III, B-IV, C-I, D-II
(4). A-III, B-II, C-IV, D-I
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Arrange the following elements in increasing order of electronegativity:
N, O, F, C, Si
Choose the correct answer from the options given below
(1). O < F < N < C < Si
(2). F < O < N < C < Si
(3). Si < C < N < O < F
(4). Si < C < O < N < F
N, O, F, C, Si
Choose the correct answer from the options given below
(1). O < F < N < C < Si
(2). F < O < N < C < Si
(3). Si < C < N < O < F
(4). Si < C < O < N < F
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
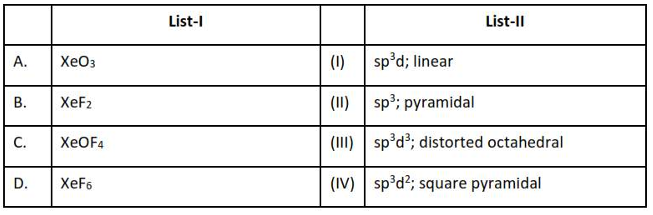
9. Match List-I with List-II.
Choose the correct answer from the options
given below:
(1). A-IV, B-I, C-II, D-III
(2). A-III, B-I, C-IV, D-II
(3). A-III, B-IV, C-I, D-II
(4). A-II, B-IV, C-I, D-III
| List-I | List-II |
|---|---|
| A. Coke | I. Carbon atoms are sp3 hybridised |
| B. Diamond | II. Used as a dry lubricant |
| C. Fullerene | II. Used as a reducing Oagent |
| D. Graphite | IV. Cage like molecules |
(1). A-IV, B-I, C-II, D-III
(2). A-III, B-I, C-IV, D-II
(3). A-III, B-IV, C-I, D-II
(4). A-II, B-IV, C-I, D-III
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statements are true?
A. Unlike \(G_a\) that has a very high melting point, \(G_s\) has a very low melting point.
B. On Pauling scale, the electronegativity values of N and Cl are not the same.
C. Ar, K+, C-, Ca2+, and S2- are allisoelectronic species.
D. The correct order of the first ionization enthalpies of Na, Mg, Al, and Si is Si > Al > Mg > Na.
E. The atomic radius of Cs is greater than that of Li and Rb.
Choose the correct answer from the options given below:
(1). A, C, and E only
(2). A, B, and E only
(3). C and E only
(4). C and D only
A. Unlike \(G_a\) that has a very high melting point, \(G_s\) has a very low melting point.
B. On Pauling scale, the electronegativity values of N and Cl are not the same.
C. Ar, K+, C-, Ca2+, and S2- are allisoelectronic species.
D. The correct order of the first ionization enthalpies of Na, Mg, Al, and Si is Si > Al > Mg > Na.
E. The atomic radius of Cs is greater than that of Li and Rb.
Choose the correct answer from the options given below:
(1). A, C, and E only
(2). A, B, and E only
(3). C and E only
(4). C and D only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements :
Statement I : Like nitrogen that can form ammonia, arsenic can form arsine.
Statement II : Antimony cannot form antimony pentoxide.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Statement I is incorrect but Statement II is correct
(2). Both Statement I and Statement II are correct
(3). Both Statement I and Statement II are incorrect
(4). Statement I is correct but Statement II is incorrect
Statement I : Like nitrogen that can form ammonia, arsenic can form arsine.
Statement II : Antimony cannot form antimony pentoxide.
In the light of the above statements, choose the most appropriate answer from the options given below:
(1). Statement I is incorrect but Statement II is correct
(2). Both Statement I and Statement II are correct
(3). Both Statement I and Statement II are incorrect
(4). Statement I is correct but Statement II is incorrect
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
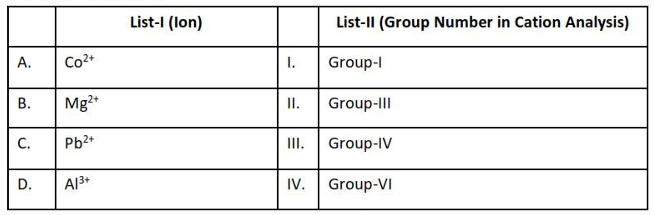
Match List - I with List - II
Choose the correct answer from the options given below :
(1). A-IV, B-II, C-I, D-III
(2). A-II, B-I, C-IV, D-III
(3). A-ll, B-I, C-III, D-IV
(4). A-IV, B-II, C-III, D-I

Choose the correct answer from the options given below :
(1). A-IV, B-II, C-I, D-III
(2). A-II, B-I, C-IV, D-III
(3). A-ll, B-I, C-III, D-IV
(4). A-IV, B-II, C-III, D-I
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which among the following electronic configurations belong to main group elements?
A. [Ne]3s1
B. [Ar]3d3 4s2C. [Kr]4d10 5s2 5p5D. [Ar]3d104s1
E. [Rn]5f0 6d27s2
Choose the correct answer from the option given below :
(1). A, C and D only
(2). B and E only
(3). A and C only
(4). D and E only
A. [Ne]3s1
B. [Ar]3d3 4s2C. [Kr]4d10 5s2 5p5D. [Ar]3d104s1
E. [Rn]5f0 6d27s2
Choose the correct answer from the option given below :
(1). A, C and D only
(2). B and E only
(3). A and C only
(4). D and E only
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The element expected to form largest ion to achieve the nearest noble gas configuration is
(1). F
(2). N
(3). Na
(4). O
(1). F
(2). N
(3). Na
(4). O
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Taking stability as the factor, which one of the
following represents correct relationship?
(1). Inl3 > InI
(2). AlCl > AlCl3
(3). Tll > TII3
(4). TICl3 > TICI
(1). Inl3 > InI
(2). AlCl > AlCl3
(3). Tll > TII3
(4). TICl3 > TICI
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II
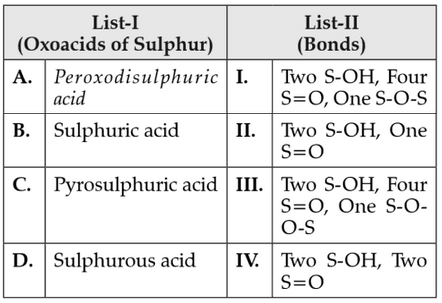
Choose the correct answer from the options given below:
(1). A-IIL, B-IV, C-I, D-II
(2). A-I, B-LII, C-IV, D-II
(3). A-IIL, B-IV, C-II, D-I
(4). A-I, B-IIL, C-IL, D-IV

Choose the correct answer from the options given below:
(1). A-IIL, B-IV, C-I, D-II
(2). A-I, B-LII, C-IV, D-II
(3). A-IIL, B-IV, C-II, D-I
(4). A-I, B-IIL, C-IL, D-IV
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Identify the incorrect statement from the following
(1). Alkali metals react with water to form their hydroxides.
(2). The oxidation number of K in KO2 is +4.
(3). Ionisation enthalpy of alkali metals decreases from top to bottom in the group.
(4). Lithium is the strongest reducing agent among the alkali metals.
(1). Alkali metals react with water to form their hydroxides.
(2). The oxidation number of K in KO2 is +4.
(3). Ionisation enthalpy of alkali metals decreases from top to bottom in the group.
(4). Lithium is the strongest reducing agent among the alkali metals.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
The IUPAC name of an element with atomicnumber 119 is
(1). ununennium
(2). unnilennium
(3). unununnium
(4). ununoctium
(1). ununennium
(2). unnilennium
(3). unununnium
(4). ununoctium
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements: one is labelledas Assertion (A) and the other is labelled asReason (R).
Assertion (A) : ICI is more reactive than I2.
Reason (R): I-CI bond is weaker than I-I bond.
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
(1). Both (A) and (R) are correct and (R) is the correctexplanation of (A).
(2). Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(3). (A) is correct but (R) is not correct.
(4). (A) is not correct but (R) is correct.
Assertion (A) : ICI is more reactive than I2.
Reason (R): I-CI bond is weaker than I-I bond.
In the light of the above statements, choose themost appropriate answer from the options givenbelow:
(1). Both (A) and (R) are correct and (R) is the correctexplanation of (A).
(2). Both (A) and (R) are correct but (R) is not the correct explanation of (A).
(3). (A) is correct but (R) is not correct.
(4). (A) is not correct but (R) is correct.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Given below are two statements:
Statement I : The boiling points of the following hydrides of group 16 elements increases in the order
H2O < H2S < H2Se < H2Te.
Statement II: The boiling points of these hydrides increase with increase in molar mass.
In the light of the above statements, choose themost appropriate answer from the options givenbelow :
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but Statement II isCorrect.
Statement I : The boiling points of the following hydrides of group 16 elements increases in the order
H2O < H2S < H2Se < H2Te.
Statement II: The boiling points of these hydrides increase with increase in molar mass.
In the light of the above statements, choose themost appropriate answer from the options givenbelow :
(1). Both Statement I and Statement II are correct.
(2). Both Statement I and Statement II are incorrect.
(3). Statement I is correct but Statement II isincorrect.
(4). Statement I is incorrect but Statement II isCorrect.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II
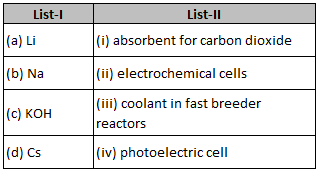
Choose the correct answer from the options givenbelow :
(1). (a) - (iv), (b) - (i), (c) – (iii), (d) - (ii)
(2). (a) -(iii), (b) – (iv), (c) – (ii), (d) -(i)
(3). (a) - (i), (b) - (iii), (c) - (iv), (d) - (i)
(4). (a)- (ii), (b) - (iii), (c) -(i), (d) - (iv)

Choose the correct answer from the options givenbelow :
(1). (a) - (iv), (b) - (i), (c) – (iii), (d) - (ii)
(2). (a) -(iii), (b) – (iv), (c) – (ii), (d) -(i)
(3). (a) - (i), (b) - (iii), (c) - (iv), (d) - (i)
(4). (a)- (ii), (b) - (iii), (c) -(i), (d) - (iv)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Match List-I with List-II.

Choose the correct answer from the options givenbelow:
(1). (a) - (iv), (b) - (i), (c) - (ii), (d) - (iii)
(2). (a)-(iii), (b)– (i), (c) - (ii), (d) - (iv)
(3). (a) - (iii), (b) - (ii), (c) - (iv), (d) - (iii)
(4). (a) - (ii), (b) - (iii), (c) - (iv), (d) - (i)

Choose the correct answer from the options givenbelow:
(1). (a) - (iv), (b) - (i), (c) - (ii), (d) - (iii)
(2). (a)-(iii), (b)– (i), (c) - (ii), (d) - (iv)
(3). (a) - (iii), (b) - (ii), (c) - (iv), (d) - (iii)
(4). (a) - (ii), (b) - (iii), (c) - (iv), (d) - (i)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Which of the following statement is not correctabout diborane?
(1). There are two 3-centre-2-electron bonds.
(2). The four terminal B-H bonds are two centretwo electron bonds.
(3). The four terminal Hydrogen atoms and the two Boron atoms lie in one plane.
(4). Both the Boron atoms are sp2 hybridised.
(1). There are two 3-centre-2-electron bonds.
(2). The four terminal B-H bonds are two centretwo electron bonds.
(3). The four terminal Hydrogen atoms and the two Boron atoms lie in one plane.
(4). Both the Boron atoms are sp2 hybridised.
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Zr (Z = 40) and Hf (Z = 72) have similar atomic and ionic radii because of :
(1). belonging to same group
(2). diagonal relationship
(3). lanthanoid contraction
(4). having similar chemical properties
(1). belonging to same group
(2). diagonal relationship
(3). lanthanoid contraction
(4). having similar chemical properties
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it ?
(1). HF < HCl < HBr < HI : Increasing acidic strength
(2). H2O < H2S < H2Se < H2Te : Increasing pKa values
(3). NH3 < PH3 < AsH3 < SbH3 : Increasing acidic character
(4). CO2 < SiO2 < SnO2 < PbP2 : Increasing oxidizing power
(1). HF < HCl < HBr < HI : Increasing acidic strength
(2). H2O < H2S < H2Se < H2Te : Increasing pKa values
(3). NH3 < PH3 < AsH3 < SbH3 : Increasing acidic character
(4). CO2 < SiO2 < SnO2 < PbP2 : Increasing oxidizing power
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
ldentify the incorrect match.
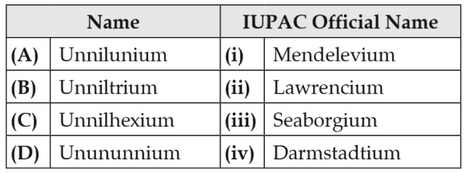
(1). (b), (ii)
(2). (c), (iii)
(3). (d), (iv)
(4). (a), (i)

(1). (b), (ii)
(2). (c), (iii)
(3). (d), (iv)
(4). (a), (i)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
For the second period elements the correct increasing order of first ionisation enthalpy is
(1). Li < B < Be < C < O < N < F < Ne
(2). Li < B < Be < C < N < O < F < Ne
(3). Li < Be < B < C < O < N < F < Ne
(4). Li < Be < B < C < N < O < F < Ne
(1). Li < B < Be < C < O < N < F < Ne
(2). Li < B < Be < C < N < O < F < Ne
(3). Li < Be < B < C < O < N < F < Ne
(4). Li < Be < B < C < N < O < F < Ne
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Among CaH2 , BeH2, BaH2 , the order of ionic character is
(1). BaH2 < BeH2 < CaH2
(2). BeH2 < BaH2 < CaH2
(3). CaH2 < BeH2 < BaH2
(4). BeH2 < CaH2 < BaH2
(1). BaH2 < BeH2 < CaH2
(2). BeH2 < BaH2 < CaH2
(3). CaH2 < BeH2 < BaH2
(4). BeH2 < CaH2 < BaH2
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
Magnesium reacts with an element (X) to form an ionic compound. If the ground state electronic configuration of (X) is 1s2 2s2 2p3, the simplest formula for this compound is
(1). Mg3X2
(2). Mg2X
(3). MgX2
(4). Mg2X3
(1). Mg3X2
(2). Mg2X
(3). MgX2
(4). Mg2X3
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
In which of the following options the order of arrangement does not agree with the variation of property indicated against it ?
(1). B < C < N < O (increasing first ionisation enthalpy)
(2). I < Br < Cl < F (increasing electron gain enthalpy)
(3). Li < Na < K < Rb (increasing metallic radius)
(4). Al3+ < Mg2+ < Na+ < F- (increasing ionic size)
(1). B < C < N < O (increasing first ionisation enthalpy)
(2). I < Br < Cl < F (increasing electron gain enthalpy)
(3). Li < Na < K < Rb (increasing metallic radius)
(4). Al3+ < Mg2+ < Na+ < F- (increasing ionic size)
Number of Attempts: 03
Correct Attempts: 01
Time Taken: 00:15
Average Time: 00:05
