Home >> Topics >> Dual nature of radiation and matter
Unattempted Questions
Questions Available: 17
Solution
Solution
Solution
Solution
Year: 2024
Topic: Dual nature of radiation and matter
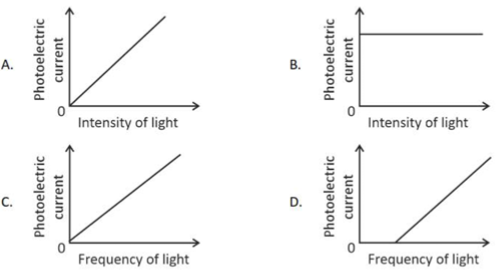
5.
If c is the velocity of light in free space, the correct statements about photon among the following are:
A. The energy of a photon is E = hv.
B. The velocity of a photon is c.
C. The momentum of a photon, p = hv/c.
D. In a photon-electron collision, both total energy and total momentum are conserved.
E. Photon possesses positive charge.
Choose the correct answer from the options given below:
A. The energy of a photon is E = hv.
B. The velocity of a photon is c.
C. The momentum of a photon, p = hv/c.
D. In a photon-electron collision, both total energy and total momentum are conserved.
E. Photon possesses positive charge.
Choose the correct answer from the options given below:
Solution
Year: 2023
Topic: Dual nature of radiation and matter
6.
The work functions of Caesium (Cs), Potassium (K) and Sodium (Na)are 2.14eV, 2.30eV and 2.75eV respectively. If incident electromagneticradiation has an incident energy of 2.20eV, which of thesephotosensitive surfaces may emit photoelectrons?
Solution
Year: 2022
Topic: Dual nature of radiation and matter
7.
When two monochromatic lights of frequency, v and \(\displaystyle \frac{v}{2}\) are incident on a photoelectric metal, their stopping potential becomes \(\displaystyle \frac{V_s}{2}\) and \(\displaystyle V_s\) respectively. The threshold frequency for this metal is
Solution
Solution
Solution
Solution
Solution
Year: 2018
Topic: Dual nature of radiation and matter
12.
An electron of mass m with an initial velocity \(\vec{v}\,=v_0\hat{i} \left( v_0\,>\,0 \right)\) enters an electric field \(\vec{E}\,=\,-\vec{E}_0 \hat{i} \left ( E_0 \,=\, constant\, >\, 0 \right )\) at t = 0. If \(λ_0\) is its de-Broglie wavelength initially, then its de-Broglie wavelength at time t is
Solution
Year: 2018
Topic: Dual nature of radiation and matter
13.
When the light of frequency \(2v_0\)
(where \(v_0\)
is threshold frequency), is
incident on a metal plate, the maximum velocity of electrons emitted is
\(v_1\)
. When the frequency of the incident radiation is increased to \(5v_0\)
, the
maximum velocity of electrons emitted from the same plate is \(v_2\)
. The
ratio of \(v_1\)
to \(v_2\)
is
Solution
Solution
Year: 2017
Topic: Dual nature of radiation and matter
15.
The photoelectric threshold wavelength of silver is \(3250 \times 10^{−10}\,m\). The velocity of the electron ejected from a silver surface by ultraviolet light of wavelength \(2536 \times 10^{−10}\,m\) is
[Given: \(h \,=\, 4.14 \times 10^{−15}\, \text{eVs}\) and \(c \,=\, 3 \times 10^8\,ms^{−1}\)]
[Given: \(h \,=\, 4.14 \times 10^{−15}\, \text{eVs}\) and \(c \,=\, 3 \times 10^8\,ms^{−1}\)]
Solution
Solution
Year: 2016
Topic: Dual nature of radiation and matter
17.
When a metallic surface is illuminated with radiation of wavelength \(\lambda\), the stopping potential is \(\text{V}\). If the same surface is illuminated with radiation of wavelength \(2\lambda\), the stopping potential is \(\displaystyle \frac{V}{4}\).The threshold wavelength for the metallic surface is