Home >> Topics >> Kinetic Theory
Unattempted Questions
Questions Available: 9
Solution
Solution
Solution
Solution
Solution
Solution
Solution
Solution
Year: 2016
Topic: Kinetic Theory
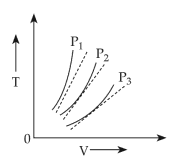
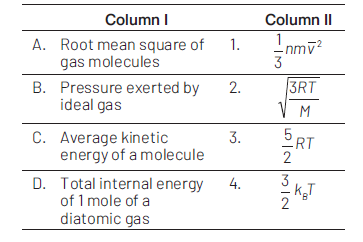
9.
The molecules of a given mass of a gas have r.m.s. velocity of \(200\, \text{ms}^{−1}\) at \(27^\circ \text{C}\) and \(1.0 \times 10^5 \text{Nm}^{−2}\) pressure. When the temperature and pressure of the gas are respectively, \(127^ \circ \text{C}\) and \(0.05 \times 10^5 \text{Nm}^{ −2}\) , the rms velocity of its molecules in \(\text{ms}^{ −1}\) is