Home >> Topics >> Thermodynamics
Unattempted Questions
Questions Available: 19
Solution
Solution
Year: 2025
Topic: Thermodynamics
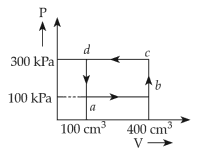
3.
Two gases A and B are filled at the same pressure in separate cylinders with movable pistons of radius \(\text{r}_\text{A}\) and \(\text{r}_\text{B}\), respectively. On supplyig an equal amount of heat to both the systems reversibly under constant presurre, the pistons of gas A nd B are displaced by 16 cm and 9 cm, respectively. If the change in their internal energy is the same, then the ration \(\displaystyle \frac{\text{r}_\text{A}}{\text{r}_\text{B}}\) is equal to
Solution
Year: 2025
Topic: Thermodynamics
4.
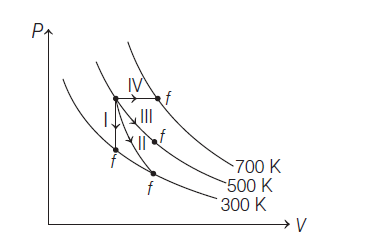
A container has two chambers of volumes \(\text{V}_1\,=\, 2\,\text{litres}\) and \(\text{V}_2\,=\, 3\,\text{litres}\) separated by a partition made of a thermal insulator. The chambers contain n1 = 5 and n2 = 4 moles of ideal gas at pressure p1 = 1 and p2 = 2 atm, respectively. When the partition is removed, the mixture attains equilibrium pressure of
Solution
Year: 2025
Topic: Thermodynamics
5.
An oxygen cylinder of volume 30 litre has 18.20 moles of oxygen. After some oxygen is withdrawn from the cylinder, its gauge pressure drops to 11 atmospheric pressures at temperature \(27^\circ\ \text{C}\). The mass of oxygen withdrawn from the cylinder is nearly equal to:
[Given, \(\text{R}\,=\, \frac{100}{12}\text{J mol}^{-1}\text{K}^{-1}\) and molecular mass of \(\text{O}_2\,=\, 32\), 1 atm pressure \(=\,1.01 \times 10^5 \text{N/m}\)]
[Given, \(\text{R}\,=\, \frac{100}{12}\text{J mol}^{-1}\text{K}^{-1}\) and molecular mass of \(\text{O}_2\,=\, 32\), 1 atm pressure \(=\,1.01 \times 10^5 \text{N/m}\)]
Solution
Solution
Solution
Solution
Year: 2021
Topic: Thermodynamics
9.
A cup of coffee cools from \(90^\circ C\) to \(80^\circ C\) in t minutes, when the room temperature is \(20^\circ C\). The time taken by a similar cup of coffee to cool from \(80^\circ C\) to \(60^\circ C\) at a room temperature same at \(20^\circ C\) is
Solution
Year: 2020
Topic: Thermodynamics
10.
Two cylinders A and B of equal capacity are connected to each other via a stop cock. A contains an ideal gas at standard temperature and pressure. B is completely evacuated. The entire system is thermally insulated. The stop cock is suddenly opened. The process is